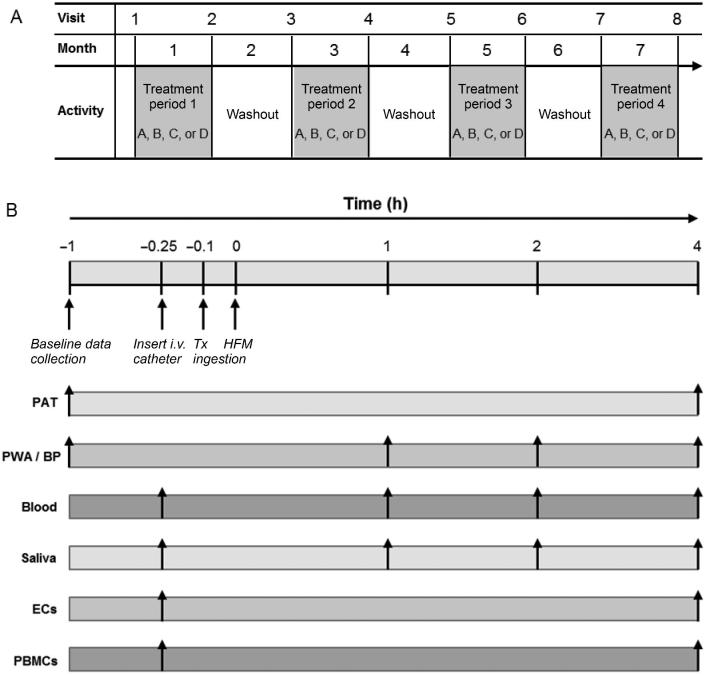
FIGURE 2.
(A) Overall study design and schedule of participant study visits. After enrollment, participants were randomized to receive four 70 mL treatments in random order: 1) placebo (PBO), 2) red beetroot juice (RBJ), 3) placebo + nitrate (PBO + NIT), and 4) nitrate-free RBJ (NF-RBJ). Each treatment period consisted of 2 postprandial challenges (i.e. first and last day of each 4-wk treatment period), followed by 4 wk of daily treatment consumption. Each treatment period was separated by a 4-wk washout period. Participants were enrolled in the trial for an 8-mo period. (B) Schematic of the test day timeline for data collection and measurements. Participants were randomly assigned to treatments A, B, C, or D for each treatment period in random order. BP, blood pressure; EC, endothelial cell; HFM, high-fat meal; i.v., intravenous; PAT, peripheral arterial tonometry; PBMC, peripheral blood mononuclear cell; PWA, pulse wave analysis; Tx, treatment.