Figure 1. Molecular Mechanisms for the Sex-Specific Contributions of EC-MR to Cardiovascular Disease.
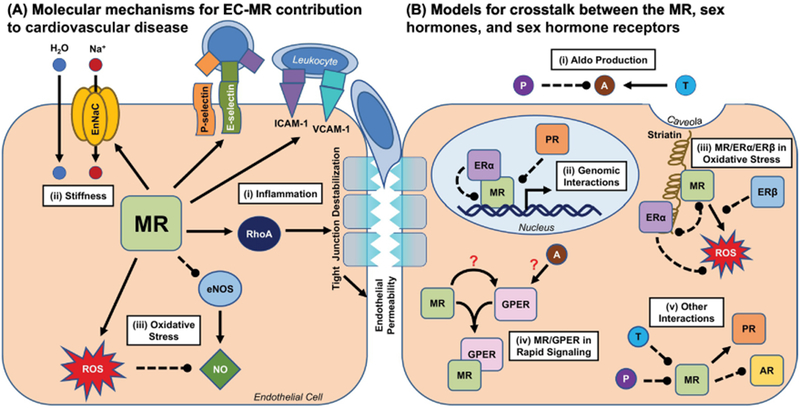
(A) The MR participates in a number of processes in ECs that may contribute to cardiovascular disease in a sex-specific manner. (i) EC-MR promotes the expression of endothelial adhesion molecules such as P- and E-selectin and ICAM-1, and this differs by sex for E-selectin and ICAM-1. This results in differential leukocyte recruitment to the vasculature in males and females. EC-MR also promotes endothelial permeability by activating RhoA, which leads to tight junction destabilization and may facilitate leukocyte trans-endothelial migration. (ii) The MR is well known to promote the expression of sodium transport proteins such as EnNaC, which in the endothelium can promote vascular stiffness. Whether this differs by sex is unclear, as all studies of EC-MR in vascular stiffness have been performed in female mice. (iii) EC-MR promotes oxidative stress in both males and females, though the mechanism for this effect may differ by sex. The ROS produced by this effect inactivate NO, thus preventing effective endothelium-dependent dilation of the underlying smooth muscle cells. This effect appears to vary by sex, arterial bed, and disease model. (B) There are several potential nodes for crosstalk between the MR and sex hormone receptors, many of which have yet to be fully explored. (i) Sex hormones may modulate production of the MR ligand Aldo at the level of the adrenal gland: testosterone may increase Aldo production, while progesterone may inhibit it. (ii) Activated ERα can bind to and inhibit the transcriptional function of the MR, which requires nuclear translocation but does not require ERα itself to bind DNA. The PR has also been demonstrated to inhibit MR transcriptional activities. (iii) The MR and ERα may compete for occupancy of striatin at the caveolar membrane, where they mediate non-genomic effects on eNOS and other rapid signaling cascades. (iv) Possible interactions between Aldo, the MR, and GPER are particularly controversial. Activation of either the MR or GPER can activate similar rapid signaling pathways, and many of these effects can be blocked by either MR inhibition or GPER inhibition. Possible models for this crosstalk include activation of GPER by MR, direct binding of Aldo to GPER, and complex formation between the MR and GPER. (v) Progesterone has been shown to bind to and inhibit the MR, and testosterone has been hypothesized to do the same. AR/MR interactions are not well characterized but may include inhibition of the AR by MR. ERβ has also been demonstrated to attenuate Aldo-induced ROS production, through unclear mechanisms. The MR may also promote PR activity. Solid arrow=positive regulation, dotted line=negative regulation; A=Aldo; AR=androgen receptor; EnNaC=endothelial epithelial sodium channel; eNOS=endothelial nitric oxide synthase; ER=estrogen receptor; GPER=G protein-coupled estrogen receptor; NO=nitric oxide; P=progesterone; PR=progesterone receptor; ROS=reactive oxygen species; T=testosterone.
