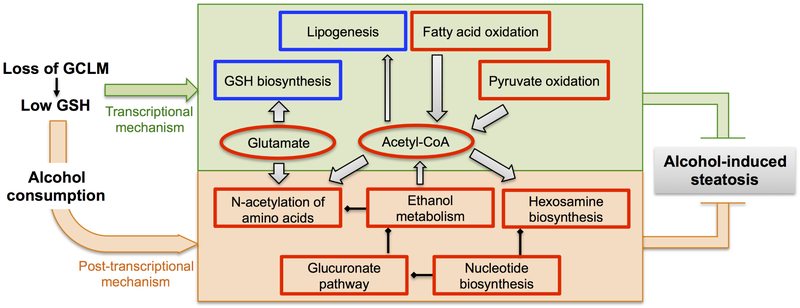
Fig. 7. Scheme of GSH deficiency-elicited reprogramming of hepatic metabolic machinery in response to alcohol consumption.
Compared with the WT liver, the GSH-deficient (due to loss of GCLM) liver exhibits intrinsic metabolic changes (green shaded area) involving the metabolism of amino acids, lipid, fatty acids, and glucose. They represent metabolic adaptations largely mediated by transcriptional mechanisms. The GSH-deficient (Gclm-KO) liver is acetyl-CoA enriched, as a result of increased production from pyruvate oxidation and fatty acid oxidation, and reduced flux to de novo lipogenesis. Following chronic alcohol consumption, these metabolic adaptations remain functional and are supplemented by unique metabolic changes occurring in the ethanol-exposed Gclm-KO liver (orange shaded area), including increased acetyl-CoA flux to N-acetylation of amino acids and hexosamine biosynthesis, elevated production of glutamate (that boosts GSH biosynthesis and serves as an alternative sink for ethanol-derived acetyl group), induction of the glucuronate pathway (that contributes to ethanol metabolism), and induction of nucleotide biosynthesis (that feeds into hexosamine biosynthesis and glucuronic acid pathways). Post-transcriptional mechanisms likely play a larger role in mediating metabolic adaptations upon ethanol exposure. Such coordinate reprograming of hepatic metabolic machinery serves to protect Gclm-KO mice against alcohol-induced steatosis. Hepatic levels of metabolites (circles) or activities of metabolic pathways (boxes) in Gclm-KO mice that are higher (red outlined) or lower (blue outlined) than WT mice are shown. Grey bands with arrows indicate metabolic flux of metabolites. Lines terminated by solid diamonds indicate cellular source for downstream metabolic pathways.