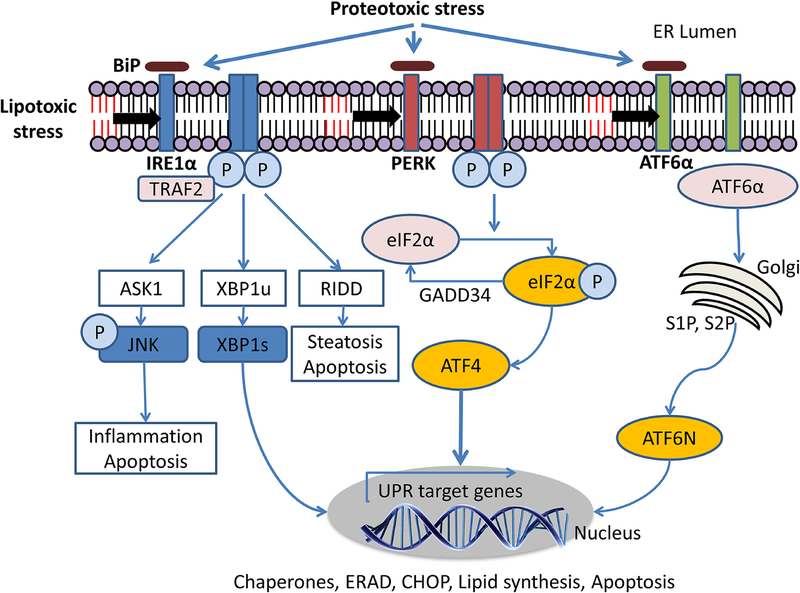
Figure 1. Proteotoxic and lipotoxic unfolded protein response signaling.
Proteotoxic stress due to the accumulation of misfolded or unfolded proteins activates the three transmembrane ER stress sensors (IRE1α, PERK, ATF6) by releasing them from BiP binding or by direct biding of the unfolded proteins to the luminal domain of the UPR sensors. Lipotoxic ER stress due to increased membrane saturation or sphingolipid accumulation is sensed by the transmembrane domains of the UPR sensors.Activated IRE1α induces splicing of XBP1. Spliced XBP1 transcriptionally upregulates genes that encode protein folding machinery, ERAD genes, and lipid synthesis pathways. PERK phosphorylates eIF2α which suppress mRNA translation leading to attenuation of protein synthesis. ATF4 is selectively translated and upregulates several transcriptional targets including CHOP. CHOP-dependent apoptosis occurs under unresolved ER stress. ATF6α translocates from the ER to the Golgi complex and cleaved by S1P and S2P. Cleaved ATF6α, termed ATF6N, transcriptionally upregulates UPR target genes. Abbreviation: IRE1α, inositol-requiring enzyme 1; PERK, PKR-like endoplasmic reticulum kinase; ATF6α, activating transcription factor 6; TRAF2, Tumor necrosis factor receptor-associated factor 2; ASK1, apoptosis-signal-regulating kinase 1; JNK, c-Jun N-terminal kinase; XBP1u, X-box binding protein 1; XBP1s, spliced X-box binding protein 1; RIDD, regulated IRE1α-dependent decay; eIF2α, eukaryotic translation initiation factor 2α; GADD34, growth arrest and DNA damage-inducible protein; ATF4, activating transcription factor 4; UPR, unfolded protein response; ERAD, endoplasmic reticulum-associated degradation; CHOP, CCAAT-enhancer-binding protein homologous protein; ATF6N, cleaved ATF6; BiP, binding immunoglobulin protein; S1P, site 1 protease; S2P, site 2 protease.