Abstract
Background
High flow nasal cannula (HFNC) is known to increase global ventilation volume in healthy subjects. We sought to investigate the effect of HFNC on global and regional ventilation patterns in patients with hypoxia.
Methods
Patients were randomized to receive one of two oxygen therapies in sequence: nasal cannula (NC) followed by HFNC or HFNC followed by NC. Global and regional ventilation was assessed using electric impedance tomography.
Results
Twenty-four patients participated. Global tidal variation (TV) in the lung was higher during HFNC (NC, 2,241 ± 1,381 arbitrary units (AU); HFNC, 2,543 ± 1,534 AU; P < 0.001). Regional TVs for four iso-gravitational quadrants of the lung were also all higher during HFNC than NC. The coefficient of variation for the four quadrants of the lung was 0.90 ± 0.61 during NC and 0.77 ± 0.48 during HFNC (P = 0.035). Within the four gravitational layers of the lung, regional TVs were higher in the two middle layers during HFNC when compared to NC. Regional TV values in the most ventral and dorsal layers of the lung were not higher during HFNC compared with NC. The coefficient of variation for the four gravitational layers of the lung were 1.00 ± 0.57 during NC and 0.97 ± 0.42 during HFNC (P = 0.574).
Conclusions
In patients with hypoxia, ventilation of iso-gravitational regions of the lung during HFNC was higher and more homogenized compared with NC. However, ventilation of gravitational layers increased only in the middle layers. (Clinical trials registration number: NCT02943863).
Keywords: electric impedance, oxygen inhalation therapy, pulmonary ventilation
Introduction
Oxygen therapy for patients with hypoxia varies according to the severity of hypoxia, and ranges from low-flow oxygen therapy to mechanical ventilation. Mechanical ventilation is thought to be the most effective method, but can cause serious complications including barotrauma and ventilator-associated pneumonia [1]. For this reason, mechanical ventilation is best avoided in cases of non-severe hypoxia. In these patients, commonly-used methods for oxygen therapy include low-flow (nasal cannula, oxygen masks) and high-flow oxygen (Venturi mask) systems. Although these delivery systems have a long history of use, inherent drawbacks exist. For example, oxygen concentration is known to be unreliable in low-flow systems, while the maintenance of sufficient humidification and heat can be challenging in high-flow systems. Due to these shortcomings, improved oxygen delivery methods are needed for the safe and effective treatment of patients with hypoxia.
As an alternative to existing methods, high flow nasal cannula (HFNC) using heated and humidified gas has emerged as a novel oxygen delivery method. HFNC has been historically used for the treatment of hypoxic neonatal and pediatric patients [2]. Recently, experiences have produced some positive results with a reduced risk of tracheal intubation rates and mortality shown in adult patients with hypoxia [3].
The benefits of HFNC are thought to arise from the effects of the heated and humidified gas, the high flow rate (which minimizes entrainment of room air), increased ventilation efficiency via the elimination of nasopharyngeal dead space, the positive end-expiratory pressure (PEEP) effect and reductions in paradoxical abdominal movement [4]. In terms of the effect on lung volume, the global and regional ventilation of the lung is known to increase during HFNC use in postoperative and healthy subjects, which is thought to be a result of the PEEP effect [5,6]. However, it remains to be determined how regional ventilation in patients with hypoxia during HFNC compares with conventional nasal cannula (NC).
Materials and Methods
Participants
Eligible patients were receiving oxygen via NC at 6 L/min or less due to hypoxia, which was defined as an oxygen saturation (SaO2) of less than 90% in room air. Patients with unstable vital signs, severe hypoxia, impending respiratory failure, symptomatic ischemic heart disease, and those who were unable to use HFNC for reasons such as nasopharyngeal deformities were excluded. The eligibility and exclusion criteria are shown in Table 1.
Table 1.
Eligibility and exclusion criteria
| Criteria for inclusion and exclusion |
|---|
| Eligibility criteria |
| ∙ Age >20 years |
| ∙ Subjective dyspnea in room air |
| ∙ SaO2 <90% in room air |
| ∙ Oxygen requirement for NC <6 L/min |
| Exclusion criteria |
| ∙ Unstable vital sign |
| - Systolic blood pressure <90 mmHg |
| - Diastolic blood pressure <60 mmHg |
| - Heart rate >120 bpm |
| - Respiratory rate >30 bpm |
| - Persistent dyspnea under oxygen therapy using NC |
| ∙ Severe hypoxia |
| - PaO2/FiO2 <200 mmHg |
| ∙ Unable to cooperate |
| - Delirium |
| - Reduced cognitive function |
| ∙ Symptomatic ischemic heart disease |
| ∙ Use of accessory muscle under oxygen therapy using NC |
| ∙ Impracticality of HFNC or NC application |
| - Facial deformity |
SaO2: oxygen saturation; NC: nasal cannula; HFNC: high flow nasal cannula.
Study Design
This was a randomized, controlled, cross-over, within-subject comparison study occurring from October 1, 2014 to February 28, 2015. The study was conducted at a tertiary referral medical center, Asan Medical Center in Seoul, Korea. The Institutional Review Board of Asan Medical Center approved the study protocol (IRB No. 2014–0763), and all participating patients provided written informed consent. The study protocol was also registered and approved in clinical registration system of U.S. National Institutes of Health (registration number: NCT02943863).
Patients received both of the two types of oxygen therapy in turn: NC followed by HFNC or HFNC followed by NC. Assignment to one of these sequences occurred using a predefined randomization table generated by Microsoft Excel (Microsoft Redmond, WA, USA). Patients received each type of oxygen therapy for 20 minutes. One of the authors (DHL) and a respiratory therapist attended at the bedside executing the study and monitoring the subjects. Changeover from one oxygen therapy to the other oxygen therapy was done in less than 2 minutes. During the study, vital signs including oxygen saturation were monitored. Electric impedance tomography (EIT) data were recorded at the end of the 20 minutes session of both oxygen therapies using a separate regional ventilation monitor (PulmoVista 500; Dräger Medical, Lübeck, Germany). The data samples for analysis were collected at the end of each type of oxygen therapy, with patients also answering a questionnaire on perceived discomfort. The overall study design is shown in Figure 1.
Figure 1.
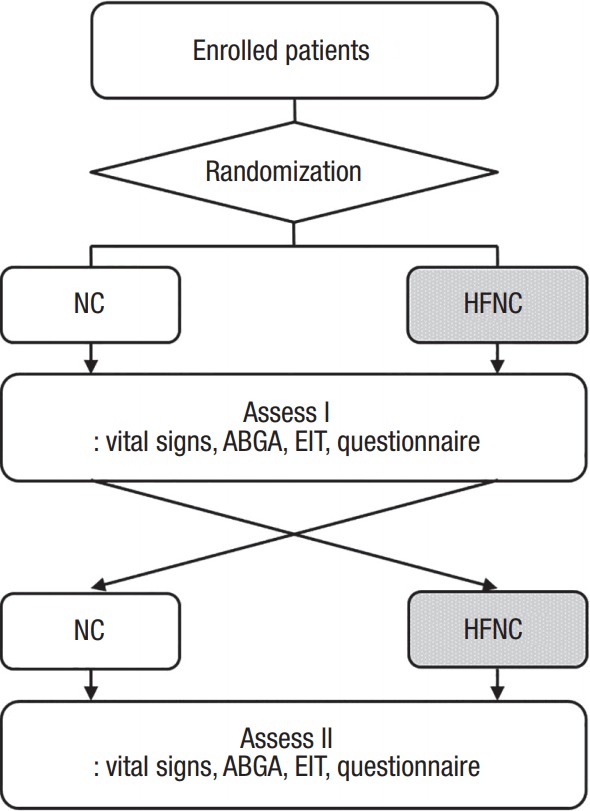
Overall study design. Enrolled patients received both types of oxygen therapy, nasal cannula (NC) and high flow nasal cannula (HFNC). The sequence of oxygen therapy, NC followed by HFNC or HFNC followed by NC, was randomized with each type of oxygen therapy lasting for 20 minutes. Efficacy and subjective discomfort associated with each treatment were assessed at the end of each therapy type. ABGA: arterial blood gas analysis; EIT: electric impedance tomography.
Oxygen Therapy
An OmniOx System (MEK-ICS, Paju, Korea) was used to administer HFNC. Initially, the gas flow rate was set at 35 L/min with FiO2 at 35%. These parameters were adjusted according to the discretion of the investigating physician to ensure optimal oxygenation and comfort for the subject. The settings were again confirmed by the attending physician prior to the initiation of the study. PEEP in the nasopharynx was monitored by pressure sensor embedded in the OmniOx during HFNC and recorded. Subjective comfort was assessed with a questionnaire consisting of 10 questions. Patients answered on a numeric rating scale from 1 to 5, with a score of 1 representing “not at all,” 2 “slightly so,” 3 “moderately so,” 4 “quite a lot,” and 5 “extreme.” We chose score 4 or higher as clinically significant level of discomfort. The specific questions answered by the patients in the questionnaire are shown in the Results section.
Electric Impedance Tomography
For the EIT study, patients were either seated or in the semi-Fowler’s position. An electrode belt containing 16 electrodes was placed around the chest wall at a location two or three fingers below the nipples with one reference electrode on the midline of the epigastric area. EIT was recorded for the whole time of two consecutive oxygen therapies, for more 40 minutes. EIT was measured at three time frames of each oxygen therapy (at 1, 2, and 3 minutes before the end of each oxygen therapy) and averaged. The oxygen therapy change process took only several minutes, which was performed by well-trained respiratory therapists and the next oxygen device was in prepared condition. The raw EIT data was recorded on a memory card device and measured using EIT analysis software (EIT Data Analysis Tool 6.1, Dräger Medical).
Changes in the impedance measured on the body surface resulting from the changes in ventilation were filtered and presented as tidal variation (TV) using arbitrary units (AU), which has been accepted to correlate strongly with actual tidal volume. A dynamic image showing the change in impedance with each breath was displayed on a monitor as cross sectional-images, with the region of interest (ROI) to be defined within the image. We equally divided the cross section of each thorax into four ROIs as iso-gravitational quadrants and four dorso-ventral gravitational layers (Figure 2). ROIs were also evaluated as dichotomous divisions (right and left regions, ventral and dorsal layers). Coefficients of variation (CoV) for the four quadrants and four layers were calculated as heterogeneity index values for regional ventilation.
Figure 2.
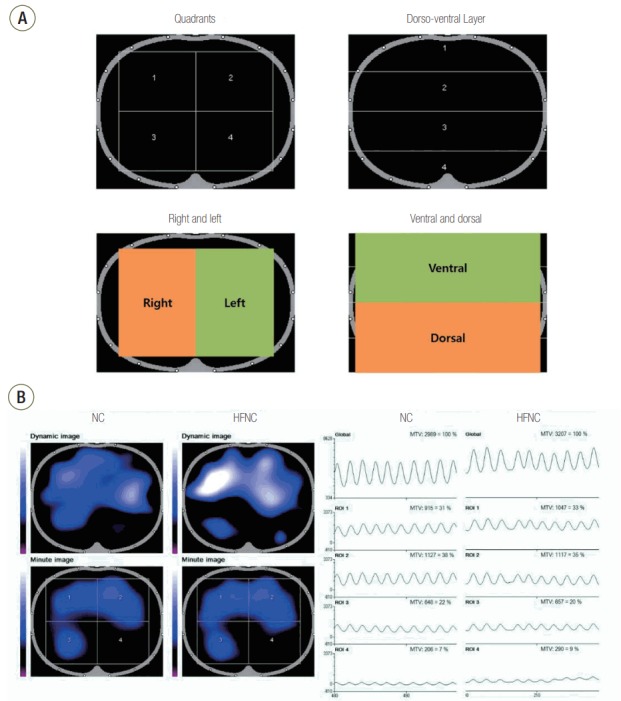
Region of interest (ROI) set showing cross-sectional images and a typical electric impedance tomography image example. (A) ROI was set as quadrants or layers and tidal variations for each ROI were provided. Comparisons of right versus left and top versus bottom were also performed. (B) Dynamic image showing change in impedance for each breath is shown at top left. Tidal variations in the global section area and each ROI are shown on the right side with quantitative graphs. The presenting case shows reduced ventilation in the left dependent portion (ROI 4). The tidal variation was significantly higher during oxygen therapy with high flow nasal cannula (HFNC), especially in ROI 1. NC: nasal cannula; MTV: mean tidal variation.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation and categorical variables as numbers with percentages. Data between HFNC and NC were compared using paired t-tests for continuous variables and chi-square tests for categorical variables. CoV was calculated as standard deviation divided by mean value. All statistical comparisons were two-sided and P < 0.05 was used as the criteria for statistical significance. Statistical analyses were performed using the IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Twenty-six patients provided written informed consent to participate in the study, with two later withdrawing their consent. The remaining 24 patients all completed the study. The mean age of the 24 patients was 67.9 ± 13.4 years, with mean body mass index of 24.0 ± 2.9 kg/m2. Fifteen of the patients (62.5%) were male. The primary causes of hypoxia in the patients were pneumonia in nine patients (37.5%), acute exacerbation of chronic obstructive pulmonary disease in three patients (12.5%), acute pulmonary edema in three patients (12.5%), sepsis in five patients (20.8%), pleural effusion in two patients (8.3%), asthma in one patient (4.2%) and hemoptysis in one patient (4.2%). Prior to the study, the patients were all receiving oxygen therapy using NC, with a flow rate of 3.0 ± 1.6 L/min. The baseline characteristics and comorbidities of the subjects are shown in Table 2.
Table 2.
Baseline characteristics of the study participants
| Characteristic | Value |
|---|---|
| Baseline characteristic | |
| Age (yr) | 67.9 ± 13.4 |
| Male sex | 15 (62.5) |
| Body mass index (kg/m2) | 24.0 ± 2.9 |
| Reason for admission | |
| Pneumonia | 9 (37.5) |
| Acute exacerbation of COPD | 3 (12.5) |
| Pulmonary edema | 3 (12.5) |
| Sepsis | 5 (20.8) |
| Pleural effusion | 2 (8.3) |
| Asthma | 1 (4.2) |
| Hemoptysis | 1 (4.2) |
| Comorbidity | |
| Hypertension | 14 (58.3) |
| Diabetes mellitus | 9 (37.5) |
| Chronic kidney disease | 4 (16.7) |
| Tuberculosis-damaged lung | 3 (12.5) |
| COPD | 6 (25.0) |
| Malignancy | 7 (29.2) |
| Route of admission | |
| Outpatient department | 2 (8.3) |
| Emergency room | 21 (87.5) |
| Transfer from another hospital | 1 (4.2) |
| Oxygen therapy | |
| NC (L/min) | 3.0 ± 1.6 |
| Laboratory result | |
| White blood cell count (/µl) | 10,388 ± 4,895 |
| Hemoglobin (g/dl) | 9.4 ± 1.8 |
| Platelet count (×103/µl) | 276 ± 355 |
| Arterial blood gas analysis | |
| pH | 7.46 ± 0.04 |
| O2 therapy with NC | |
| pCO2 (mmHg) | 38.2 ± 7.8 |
| pO2 (mmHg) | 104.4 ± 30.4 |
| HCO3 (mmHg) | 27.1 ± 4.7 |
| PaO2/FiO2 ratio | 332.9 ± 112.9 |
Values are presented as mean ± standard deviation or number (%).
COPD: chronic obstructive pulmonary disease; NC: nasal cannula.
Thirteen patients received HFNC first, followed by NC, and 11 patients received their therapy in the reverse order. Mean flow rate of HFNC was 34.2 ± 4.1 L/min and mean FiO2 was 35.6% ± 1.6%. After initiation of HFNC therapy, the setting value was changed in four patients. One patient complaint of headache and flow rate of HFNC was lowered to 15 L/min. Although the lower flow rate was well-tolerated, the patients did not want to increase the flow rate again. The patient received HFNC treatment with 15 L/min and FiO2 of 35% for 20 minutes. FiO2 of HFNC was set to 40% at the beginning of HFNC therapy in three patients by decision of attending physician, although the patients did not exhibit significant hypoxia or dyspnea. They received HFNC with FiO2 40% for 20 minutes. Mean PEEP during HFNC was 4.8 ± 0.9 cmH2O. During the two types of oxygen therapy, the respiration (NC, 20.5 ± 5.0/min; HFNC, 19.8 ± 4.9/min; P = 0.403) and heart rates (NC, 88 ± 13/min; HFNC, 88 ± 12/min; P = 0.654) were not statistically different. In regard to arterial blood gas, PaO2 levels were higher during HFNC than in NC (NC, 104.3 ± 29.0 mmHg; HFNC, 122.4 ± 28.7 mmHg; P = 0.007), whereas no significant difference in PaCO2 levels (NC, 37.4 ± 6.5 mmHg; HFNC, 37.6 ± 7.2 mmHg; P = 0.728).
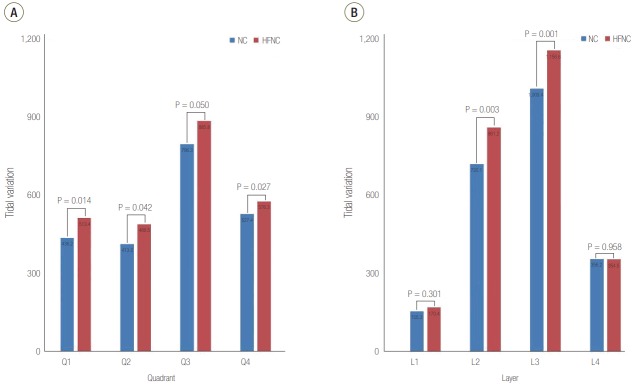
Compared with NC, the global TV of the lung was higher during HFNC (NC, 2,241 ± 1,381 AU; HFNC, 2,543 ± 1,534 AU; P < 0.001). TVs of the dorsal and ventral hemi-lung regions as well as the left and right hemilung regions were consistently higher during oxygen therapy with HFNC than with NC (Table 3). Regional TVs for the four iso-gravitational quadrants of the lung were all higher during HFNC than NC (Figure 3A). In terms of the four gravitational layers of the lung, the two middle layers were increased during HFNC compared with NC (Figure 3B). However, regional TVs in the most ventral and most dorsal layers of the lung were not statistically different from NC.
Table 3.
Comparison of tidal variation of NC versus HFNC in dorsal, ventral, left, and right regions of interest
| Variable | NC (AU) | HFNC (AU) | Percent change (%) | P-value |
|---|---|---|---|---|
| Overall | 2,241 ± 1,381 | 2,543 ± 1,534 | 15.6 ± 16.9 | <0.001 |
| Dorsal | 1,366 ± 1,084 | 1,511 ± 1,198 | 12.0 ± 16.4 | 0.009 |
| Ventral | 875 ± 645 | 1,032 ± 727 | 52.2 ± 121.2 | 0.007 |
| Left | 941 ± 693 | 1,065 ± 730 | 28.7 ± 58.1 | 0.011 |
| Right | 1,232 ± 1,124 | 1,399 ± 1,265 | 26.7 ± 63.6 | 0.003 |
Values are presented as mean ± standard deviation.
NC: nasal cannula; HFNC: high flow nasal cannula; AU: arbitrary units.
Figure 3.
Tidal variation comparison between nasal cannula (NC) and high flow nasal cannula (HFNC). Regions of interest were set as (A) quadrants and (B) layers.
When the TV was compared as quadrants, in the ROI where the TV was minimal during NC, the increase in TV during HFNC was 37.6% (NC, 122 ± 262 AU; HFNC, 168 ± 255 AU; P = 0.035). In contrast, in the ROI with maximal TV during NC, the increase in TV during HFNC was only 7.9% (NC, 1,102 ± 956 AU; HFNC, 1,188 ± 1,077 AU; P = 0.056). The CoV for the four quadrants was 0.90 ± 0.61 during NC and 0.77 ± 0.48 during HFNC (P = 0.035). The CoV for the four layers was 1.00 ± 0.57 during NC and 0.97 ± 0.42 during HFNC (P = 0.574).
In the assessment of perceived discomfort, scores for chest discomfort (NC, 1.00 ± 0.00; HFNC, 1.33 ± 0.70; P = 0.029) and noisiness (NC, 1.08 ± 0.28; HFNC, 2.04 ± 1.08; P < 0.001) were higher during HFNC compared with NC. However, other parameters showed no difference between the two therapies (Table 4). The number of questions for discomfort scoring 4 or more, representing significant discomfort, were not statistically different between the two therapies and neither did the result with score 3.
Table 4.
Results from the questionnaire assessing subjective comfort
| Question | Mean score for subjective comfort |
Incidence of score 4 or more |
||||
|---|---|---|---|---|---|---|
| NC | HFNC | P-value | NC | HFNC | P-value | |
| Dryness in nasal cavity | 1.38 ± 0.77 | 1.46 ± 0.66 | 0.679 | 1 (4.2) | 0 | 0.609 |
| Dryness in pharynx | 1.46 ± 1.02 | 1.38 ± 0.77 | 0.739 | 3 (12.5) | 1 (4.2) | 0.609 |
| Pain in pharynx | 1.29 ± 0.75 | 1.13 ± 0.34 | 0.357 | 1 (4.2) | 0 | 1.000 |
| Headache | 1.13 ± 0.45 | 1.17 ± 0.48 | 0.747 | 0 | 0 | - |
| Chest discomfort | 1.00 ± 0.00 | 1.33 ± 0.70 | 0.029 | 0 | 0 | - |
| Noisy | 1.08 ± 0.28 | 2.04 ± 1.08 | <0.001 | 0 | 3 (12.5) | 0.234 |
| Inappropriate air flow | 2.17 ± 0.64 | 1.00 ± 0.00 | 0.213 | 1 (4.2) | 0 | 1.000 |
| Hampered | 1.42 ± 0.93 | 1.50 ± 0.83 | 0.723 | 1 (4.2) | 1 (4.2) | 1.000 |
| Strong air flow | 1.29 ± 0.81 | 1.50 ± 0.93 | 0.347 | 1 (4.2) | 1 (4.2) | 1.000 |
| Gross discomfort | 1.50 ± 0.83 | 1.88 ± 0.99 | 0.119 | 1 (4.2) | 2 (8.3) | 1.000 |
Values are presented as mean ± standard deviation or number (%). Patients answered questions relating to perceived discomfort, with a score of 1 representing “not at all,” score 2 “slightly,” score 3 “moderate,” score 4 “quite a bit,” and a score of 5 “extreme.”
NC: nasal cannula; HFNC: high flow nasal cannula.
Discussion
HFNC is emerging as an alternative to conventional oxygen delivery systems and as a noninvasive ventilation approach in selected patients. In patients with non-severe hypoxia, studies have shown that HFNC helps patients avoid intubation and results in better outcomes [7-9]. Of the few speculated advantages of HFNC, the PEEP effect is particularly attractive in that its beneficial effects may be achieved noninvasively. Increased TV has been reported in a previous study using EIT [10], with HFNC in post-cardiac surgery patients associated with significantly higher TV.
Our study confirms these findings in nonsurgical patients. In patients with hypoxia in the present study, compared with conventional NC, HFNC resulted in (1) increased global ventilation, (2) increased regional ventilation in all four iso-gravitational quadrants of the lung, with decreased heterogeneity of the regions, and (3) increased regional ventilation of the two middle gravitational layers, and no change in the gravitational heterogeneity of ventilation.
We note several interesting findings on the regional characteristics of the increased ventilation during HFNC. Firstly, regional ventilation of the iso-gravitational plane of the lung was higher, and the heterogeneity was lower compared with NC. However, regional ventilation along the dorso-ventral axis was higher only in the middle part of the lung during HFNC, while this was not the case in the most dorsal and ventral regions.
PaO2 levels were also higher during HFNC than in NC. Although FiO2 levels during both HFNC and NC cannot be precisely controlled, studies on HFNC have consistently shown improved oxygenation compared with NC or reservoir masks. Our findings provide plausible explanations for this phenomenon [8,11]. The regions with lowest ventilation during NC were receiving 37.6% more during HFNC, which likely translates into a better ventilation-perfusion ratio in the region. The decreased coefficient of variation for regional TVs, at least on the iso-gravitational plane of the lung, also supports this explanation.
In a previous study on regional lung ventilation during HFNC conducted in healthy subjects, a more homogeneous distribution during HFNC was found when compared to room air breathing [6]. To our knowledge, the present study is the first to evaluate regional ventilation characteristics during HFNC in patients with hypoxia.
Patients expressed more discomfort with HFNC in terms of chest discomfort and noisiness. However, the mean scores for HFNC were 1.33 and 2.04, respectively, which are lower than moderate in the degree of discomfort, and none of the subjects withdrew from the study due to intolerable discomfort. The respiration rate during HFNC was not lower than that during NC. This may be due to the characteristics of our subjects; baseline levels of hypoxia (PaO2 104 mmHg on mean oxygen 3.0 L/min via nasal NC) and respiration rate (mean, 20.5/min) were both non-severe. Therefore, the benefits of HFNC on respiratory mechanics may have been less pronounced in our patients compared with subjects in the previous studies.
Applications for HFNC in adult patients are rapidly expanding for clinical indications, from simple acute respiratory failure to respiratory failure from diverse etiologies such as postoperative care [12,13], postextubation [9,14,15], and possibly for mild acute respiratory distress syndrome. In previous studies, oxygen therapy using HFNC has focused on patients with moderate hypoxia [10,12-15]. In the present study, HFNC was applied in patients with a mild degree of hypoxia and who were being treated with low-flow oxygen. As this represents a highly prevalent patient group, our findings may have wider implications for HFNC at the bedside.
We tried to assess the difference of TV distribution according disease group and the in-homogeneous distribution of lung disease. However, defining the in-homogeneity was difficult to avoid being subjective. For example, with distribution of pneumonia in a patient, physicians can draw different conclusions. In addition, TV and CoV showed no significant difference between disease groups. We tried to avoid describing homogeneity or in-homogeneity of lung disease.
The present study has several limitations. First, this is a non-blinded study in a small number of patients. Blinding could not be achieved due to the distinct characteristics of each oxygen therapy. However, the order of oxygen devices was randomly allocated to patients to minimize bias. We also note a limitation in that data on global and regional ventilation was limited to a single plane of the lung, as these were assessed by EIT. Relatively lower setting of flow rate and FiO2 during HFNC can also be a limitation. Enrolled patients showed non-severe hypoxia and initial setting was set lower. No patients required increase of flow rate or FiO2 during the study. Although the lower HFNC setting could have diminished the effect of HFNC on pulmonary ventilation, the result of this study does not become less meaningful.
In conclusion, during HFNC oxygen therapy, in addition to increased global ventilation, regional ventilation and its distribution was improved compared with conventional NC.
Acknowledgments
This research was financially supported by a grant from Korea Institute for Advancement of Technology, Ministry of Trade, Industry and Energy, Republic of Korea as a Leading Industry Development Project for the Wide Area Economic Bloc in Kangwon Province (R0001120). Dräger Korea (Seoul, Korea) provided electric impedance tomography monitor (PulmoVista 500) with data analysis software.
Interim results of this research were presented at 120th annual meeting of Korean Academy of Tuberculosis and Respiratory Disease and 46th Critical Care Congress.
Footnotes
Dräger Korea (Seoul, Korea) provided electric impedance tomography monitor (PulmoVista 500) with data analysis software. The funder had no role in the design, collection, analysis or interpretation of this study. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2014;370:980. doi: 10.1056/NEJMc1400293. [DOI] [PubMed] [Google Scholar]
- 2.Manley BJ, Dold SK, Davis PG, Roehr CC. High-flow nasal cannulae for respiratory support of preterm infants: a review of the evidence. Neonatology. 2012;102:300–8. doi: 10.1159/000341754. [DOI] [PubMed] [Google Scholar]
- 3.Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–96. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 4.Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest. 2015;148:253–61. doi: 10.1378/chest.14-2871. [DOI] [PubMed] [Google Scholar]
- 5.Mündel T, Feng S, Tatkov S, Schneider H. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J Appl Physiol (1985) 2013;114:1058–65. doi: 10.1152/japplphysiol.01308.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riera J, Pérez P, Cortés J, Roca O, Masclans JR, Rello J. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care. 2013;58:589–96. doi: 10.4187/respcare.02086. [DOI] [PubMed] [Google Scholar]
- 7.Maggiore SM, Idone FA, Vaschetto R, Festa R, Cataldo A, Antonicelli F, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation: effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. 2014;190:282–8. doi: 10.1164/rccm.201402-0364OC. [DOI] [PubMed] [Google Scholar]
- 8.Sztrymf B, Messika J, Mayot T, Lenglet H, Dreyfuss D, Ricard JD. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: a prospective observational study. J Crit Care. 2012;27:324. doi: 10.1016/j.jcrc.2011.07.075. e9-13. [DOI] [PubMed] [Google Scholar]
- 9.Yoo JW, Synn A, Huh JW, Hong SB, Koh Y, Lim CM. Clinical efficacy of high-flow nasal cannula compared to noninvasive ventilation in patients with post-extubation respiratory failure. Korean J Intern Med. 2016;31:82–8. doi: 10.3904/kjim.2016.31.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. 2011;107:998–1004. doi: 10.1093/bja/aer265. [DOI] [PubMed] [Google Scholar]
- 11.Calvano TP, Sill JM, Kemp KR, Chung KK. Use of a high-flow oxygen delivery system in a critically ill patient with dementia. Respir Care. 2008;53:1739–43. [PubMed] [Google Scholar]
- 12.Parke RL, McGuinness SP, Eccleston ML. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care. 2011;56:265–70. doi: 10.4187/respcare.00801. [DOI] [PubMed] [Google Scholar]
- 13.Itagaki T, Okuda N, Tsunano Y, Kohata H, Nakataki E, Onodera M, et al. Effect of high-flow nasal cannula on thoraco-abdominal synchrony in adult critically ill patients. Respir Care. 2014;59:70–4. doi: 10.4187/respcare.02480. [DOI] [PubMed] [Google Scholar]
- 14.Tiruvoipati R, Lewis D, Haji K, Botha J. High-flow nasal oxygen vs high-flow face mask: a randomized crossover trial in extubated patients. J Crit Care. 2010;25:463–8. doi: 10.1016/j.jcrc.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 15.Rittayamai N, Tscheikuna J, Rujiwit P. High-flow nasal cannula versus conventional oxygen therapy after endotracheal extubation: a randomized crossover physiologic study. Respir Care. 2014;59:485–90. doi: 10.4187/respcare.02397. [DOI] [PubMed] [Google Scholar]