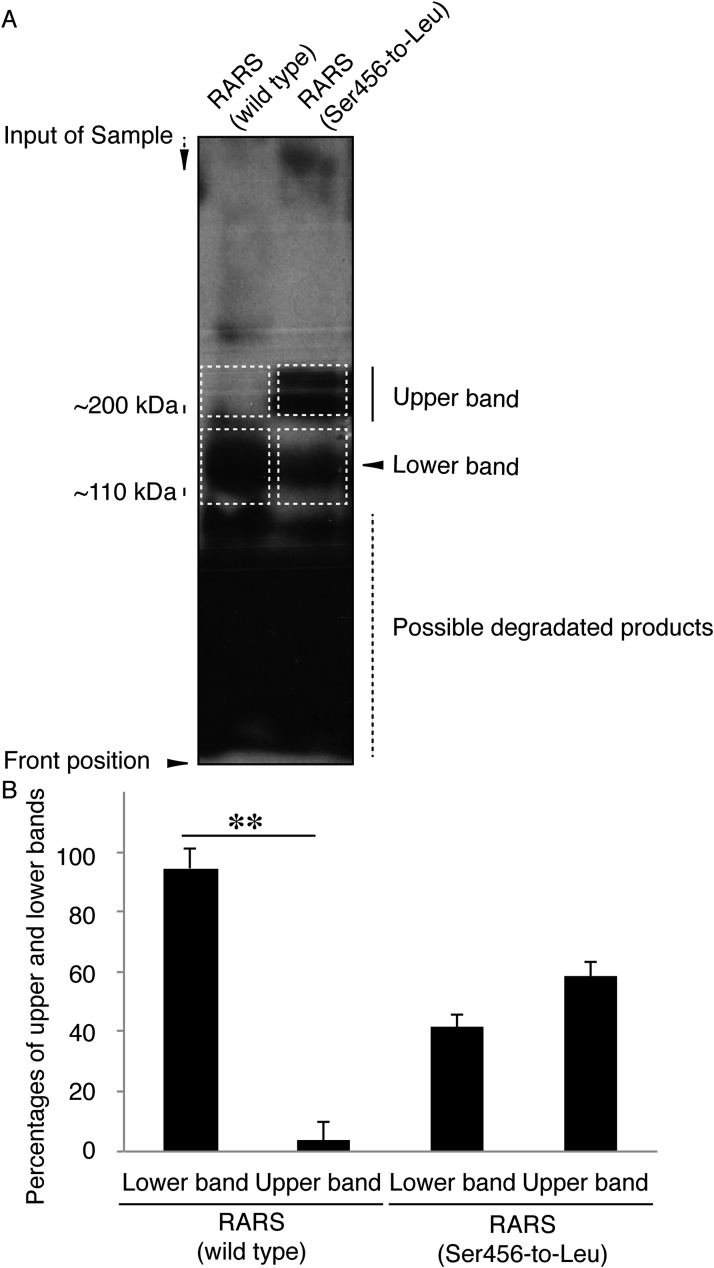
Fig. 6.
Ser456-to-Leu mutant proteins prefer to form dimeric structures in polyacrylamide electrophoresis. (A) The plasmid encoding the wild type or mutant of GFP-tagged RARS were transfected into COS-7 cells, subjected to non-denaturing polyacrylamide electrophoresis, and detected by immunoblotting with an anti-GFP antibody. The predicted molecular mass of GFP-tagged wild-type RARS is approximately 110 k and is indicated as a monomer position (lower dotted squares). GFP-tagged mutant proteins exhibit two forms with positions of approximately 110 kDa and 200 kDa markers. 200 kDa proteins correspond to the position (dotted squares) of RARS dimer. (B) Percentages of lower or upper band intensities per total band intensities are statistically shown (**, p < 0.01 of one-way analysis of variance [ANOVA] followed by a Fisher's protected least significant difference test as a post hoc comparison; n = 3 blots).