Abstract
Background
The purpose of this 6-week intervention was to test the feasibility and acceptability of implementing a telehealth-adapted Diabetes Prevention Program (DPP) at a senior center.
Methods
Older adults (n = 16) attended weekly interactive webinars. At each measurement time point, participants completed questionnaires covering lifestyle, physical activity, quality of life, and food records and wore physical activity trackers. Qualitative data were gathered from 2 focus groups inviting all 16 participants with 13 and 10 participants attending, respectively.
Results
Over 2000 senior center members were contacted, approximately 2% (n = 39) responded to the recruitment email, and 16 were recruited into the study. Retention was 75%, and attendance rates averaged 80% across the six intervention sessions. The focus group participants provided positive opinions for most program components, especially the webinar group interaction and using physical activity trackers. Suggestions for improvement included a greater focus on specific needs of older adults (i.e., adapting activities) and placing a greater emphasis on dietary strategies to prevent diabetes. Mean weight loss was 2.9% (2.7 kg [95% CI 1.6, 3.7]; p value = 0.001).
Conclusion
The feasibility of providing DPP via webinar appears to be high based on the retention and attendance rates. Similar to other behavioral interventions engaging older adults, recruitment rates were low. Acceptability was evidenced by high attendance at the intervention sessions and feedback from participants during focus group sessions. The intervention efficacy should be evaluated based on CDC criteria for program recognition in a larger scale randomized trial.
Trial registration
NCT03524404. Registered 14 May 2018—retrospectively registered. Trial protocol will be provided by the corresponding author upon request.
Keywords: Aging, Technology, Translation of evidence-based interventions, Energy balance, Nutrition, Physical activity, Diabetes Prevention Program
Background
The number of Americans 65 years or older (49.1 million as of 2016) is projected to more than double by 2060 [1]. Obesity among older adults is also increasing, from a prevalence of 31% in 2003–2004 to 35% in 2011–2012 [2]. Without adopting healthier lifestyles, 15–30% of the 87.3 million people in the USA with pre-diabetes will develop type 2 diabetes within 5 years [3]. Despite the availability of effective pharmacological [4, 5] and behavioral interventions [6–11], chronic diseases, such as type 2 diabetes and cardiovascular disease (CVD), remain major public health problems [12].
Among the 20% of participants in the Diabetes Prevention Program (DPP) who were 60 years and older (n = 648), the diet and physical activity intervention conferred a 71% reduction in risk of type 2 diabetes [13]. However, few individuals in this age group at risk for type 2 diabetes or CVD adopt healthy diets and lifestyles [14, 15].
Socioeconomically disadvantaged older adults are at particularly high risk of type 2 diabetes and its consequences, and they often do not have ready access to services designed to reduce those risks. In the Northern Manhattan Cohort Study, incidence of type 2 diabetes was twice (HR 2.0; 95% CI 1.2–3.6) as likely among blacks compared to whites [16]. In the National Health Interview Survey, people with type 2 diabetes having the lowest level of education had a 1.52 (95% CI 1.04–2.23) times greater risk of death compared to those with greatest amount of education [17].
As of April 1, 2018, Medicare reimburses for provision of a CDC-approved DPP curriculum [18]; therefore, the intervention is now more accessible to older adults. However, the DPP program is typically offered only at hospitals, YMCA’s, and health departments, limiting accessibility for older adults. Furthermore, current regulations exclude DPP programs offered online, suggesting that more data are needed to support efficacy of online programs. Adaptations of evidence-based lifestyle approaches to chronic disease prevention are needed to meet the needs of the most vulnerable, low-resource communities.
Offering an online program within senior center environments could be an innovative approach to extending the reach of the DPP to low-income seniors. The online format minimizes cost to the senior centers for implementing and administering the program. Nesting the program within senior centers provides built-in supports to reduce barriers to accessing technology.
The primary aim of this study was to test the feasibility of implementing a telehealth adaptation of the DPP within a New York City (NYC) senior center. Measures used to assess feasibility were proportion of responses from emailed invitations, proportion of those screened who were eligible for group meeting attendance, proportion of follow-up visits completed, and obtaining measurements of body weight, diet, and physical activity. Attendance at weekly sessions was used to measure acceptability. Participant focus groups were also used to qualitatively characterize intervention acceptability and feasibility. We hypothesized that the telehealth adaptation would be both feasible and acceptable for older adults within senior centers.
By first examining the feasibility of this DPP telehealth adaptation, our intention is to incorporate feedback from participants to inform the design of an effectiveness trial. As part of our examination of feasibility, we compared (as secondary outcomes) changes in weight, diet, and physical activity after implementing the telehealth program.
Methods
Participants
We recruited a purposive sample of older adults in March 2018 by sending emails to over 2000 community center members, which was supplemented by two in-person recruitment sessions at a NYC community center that offers older adults’ programs to build skills in using technology. To align with typical DPP group size and due to space constraints, the targeted sample size was 10–15 participants. Inclusion criteria included being 60 years of age or older and having a Diabetes Risk Score of greater or equal to 5 [19, 20]. The Diabetes Risk Score contains 7 items querying age, gender, history of gestational diabetes, family history, hypertension, physical activity, and weight for a total score ranging from 0 to 20. Those who lacked decisional capacity to consent were also excluded from this study. All participants provided written informed consent. This study was approved by the Institutional Review Board at New York University Langone School of Medicine. Each participant was given a $50 gift card at the conclusion of the follow-up visit.
Design
This study was a single-group design with measurements collected at baseline and 7 weeks. A certified DPP group facilitator led weekly interactive, live webinars of the first 6 of 12 core sessions of the University of Pittsburgh’s Group Lifestyle Balance 2017 Diabetes Prevention Program (see Additional file 1) [21]. Six sessions were chosen as it was believed to provide sufficient data to assess the feasibility of delivering the intervention via telehealth. The study participants gathered in person at one senior center, while the facilitator joined remotely via FaceTime for weekly hour-long sessions. The session topics included an introduction to the program, tips about eating fewer calories and fat, components of healthy eating, the benefits of and methods for tracking physical activity, and how to manage personal and social eating triggers. A research assistant measured and recorded each participant’s weight at each weekly session. The checklist for the CONSORT extension for pilot trials was completed (see Additional file 2).
Measurements
Primary measures
The proportion of responses from emailed invitations, proportion of those screened who were eligible for group meeting attendance, and proportion of follow-up visits completed were collected and analyzed to assess feasibility and acceptability. Additionally, qualitative data was collected from focus groups and used to better understand participant perceptions of the intervention.
Secondary measures
To establish feasibility of using the measures, baseline and follow-up (week 7) measurements included weight (Tanita BC-545, Tokyo, Japan), self-reported physical activity (Cardiovascular Healthy Activities Model Program for Seniors) [24], dietary intake using a 4-day food record, waist circumference, alcohol consumption (AUDIT-C) [22], smoking status (never, current, former), depression (Center for Epidemiologic Studies-Depression Scale) [23], and quality of life (RAND-36) [25, 26]. At baseline, a research assistant collected demographic information and measured subjects’ height and stored all anthropometric, demographic, and lifestyle assessment data in REDCap, a secure online web application (Research Electronic Data Capture) [27]. Physical activity trackers (Fitbit Charge 2™, Fitbit, Inc., San Francisco, CA, 2016) were provided and distributed to all participants after the second session.
Analysis
Qualitative measures
The principal investigator (JMB) led focus groups discussing the program’s acceptability and relevance for all participants in attendance after sessions 3 (n = 13) and 5 (n = 10). The rationale for conducting two focus groups among the same participants was to gather perspectives after a session on healthy eating at the midpoint and after physical activity had been introduced during sessions 4 and 5. Each focus group took place immediately after the intervention session and lasted approximately 45 min. Table 1 outlines the focus group questions asked at each focus group. Two research assistants recorded responses to the facilitator’s questions. The facilitator and two research assistants independently answered ten post-focus group summary questions and met to discuss the focus group feedback. Qualitative results were analyzed by condensing transcripts from the two research assistants, grouping results by response, and summarizing major themes.
Table 1.
Focus group questions
| • What did you think of the information in the presentations? (Amount of information, length of presentation) | |
| • What did you like the least (best) about the presentation? (General/amount of information, activity, speaker, time commitment, convenience) | |
| • For the future, if we had to make one change that would make the presentations better, what could we do to improve the presentations? | |
| • What are your thoughts on the handouts? (Tracking with materials, helpful) | |
| • When you think of “Diabetes prevention,” what are some words/phrases that come to mind? | |
| • We know that you are interested in learning about technology because you are here at Senior Planet. Do you see your peers participating in a technology-based program like this? If not, why? | |
| • How much of this information was new to you? If it was not new to you, where have you heard the information in the past? | |
| • Some of you shared ways that you track your food intake. How do you think using a nutrition smartphone application would change your participation in this program? | |
| • Fitbit has a tool where you can track each other’s activity. Do you see yourself using this to interact with other group members to see their physical activity? | |
| • If this program were repeated, what would you like to be added? |
Quantitative measures
We calculated program participation using the proportion of enrolled participants who attended each intervention session of the 6-week program. We used descriptive statistics to summarize demographics among all participants. To identify possible effect direction and magnitude, we analyzed pre-post differences of continuous scale outcomes using paired t tests and pre-post changes in categorical variables using chi-square tests. Caution is warranted in interpreting level of statistical significance of these comparisons, given the small sample size of this study. We report 95% confidence intervals for differences in means.
A research assistant (LK) analyzed food records using Nutrition Data System for Research (NDSR 2016, Nutrition Coordinating Center, University of Minnesota, MN). Self-reported physical activity was calculated by summing the hours per week of all physical activity, with moderate-intensity, exercise-related activities and then multiplying by 60 to convert to minutes per week. Physical activity tracker algorithms classified activity as lightly active, fairly active, or very active. Physical activity tracker data was considered invalid if recorded days were < 600 min, 1440 sedentary minutes, or 0 steps. Food records were considered invalid if < 4 days were recorded.
We conducted sensitivity analyses to compare demographic characteristics between all participants (n = 16) with those who completed the follow-up visit (n = 12), and to impute missing weight data using the last observation carried forward to assess whether attrition may have influenced inferences. Data analysis was conducted using SPSS (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.).
Results
The mean age of participants was 70.1 ± 5.6 years including 12 participants (75%) who were aged 65 or over (Table 2). As shown in Table 2, the sample was largely female, college educated, un-partnered, overweight, or obese. Somewhat more than half of the participants were white.
Table 2.
Baseline demographics, n = 16
| Age, mean ± SD | 70.1 ± 5.6 |
| Sex, female (%) | 11 (68.8) |
| BMI, kg/m2, mean ± SD | 31.2 ± 7.3 |
| Normal, 18 to < 25 kg/m2 (n, %) | 3 (18.8) |
| Overweight, 25 to < 30 kg/m (n, %) | 6 (37.5) |
| Obese, ≥ 30 kg/m2 (n, %) | 7 (43.8) |
| Race (n, %) | |
| White | 9 (56.3) |
| Black or African American | 6 (37.5) |
| Asian | 1 (6.3) |
| Hispanic ethnicity (n, %) | 1 (8.3) |
| Marital status (n, %) | |
| Married/living with partner | 2 (12.5) |
| Divorced | 6 (37.5) |
| Widowed | 3 (18.8) |
| Never married | 5 (31.3) |
| Education (n, %) | |
| High school graduate | 1 (6.3) |
| Some college or technical school | 5 (31.3) |
| College graduate | 10 (62.5) |
Feasibility and acceptability measures
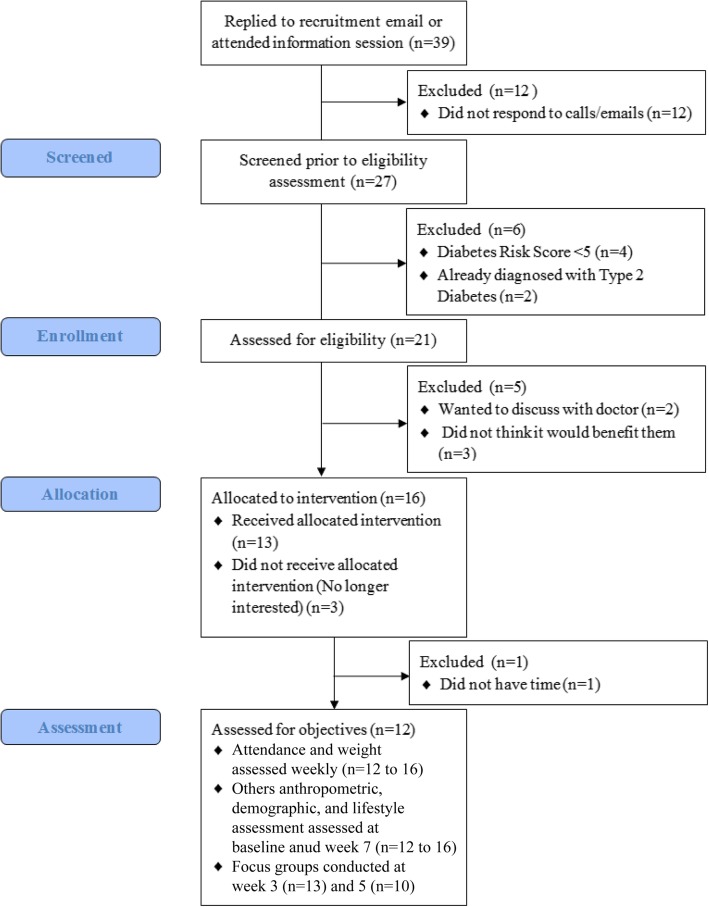
Figure 1 outlines the flow diagram of participant enrollment. Approximately 2% (n = 39) of the over 2000 senior center members responded to the recruitment email. Based on the feasibility outcome measures, 69.2% (n = 27) of total participants (n = 39) who responded to the email invitation were then screened for the feasibility study. Of the 27 participants screened, 77.8% (n = 21) of participants were eligible for group meetings. The number needed to screen to enroll 1 participant was 2.1 (Fig. 1, 27 screened/13 enrolled).
Fig. 1.
Flow diagram
As a measure of program acceptability, retention was 75%, with the majority of participants attending all intervention sessions (Table 3). All participants provided valid food records at baseline, and 12/13 (92%) provided valid food records at follow-up (Table 4). For the physical activity trackers, over 80% of participants provided valid physical activity tracker data (11/13) at week 3 follow-up, and 69% (9/13) provided valid data at week 6 follow-up (Table 5). Focus group attendees (n = 13 at the first focus group; n = 10 at the second focus group) indicated that the most favorable aspects of the program were interacting with each other, using the physical activity trackers, the resources and worksheets provided, and the session length. Unfavorable aspects of the program included the following: difficulty interacting with the health coach due to ambient noise and poor sound quality through the television, and the lack of in-depth nutritional information presented. Participants wanted “more substance” and “factual information” provided in the facilitated group sessions. A couple of participants requested the sessions be led by a dietitian, so that more advanced information could be provided beyond what is available in the handouts. In particular, program participants wanted more explanation of why reducing fat was the primary focus rather than restricting carbohydrates. Other participants’ suggestions for improvement included adapting the information for older adults by providing modified exercises and reducing environmental distractions.
Table 3.
Attendance and weight change
| Attendance | Weight, kg (mean ± SD) | Weight change from baseline | |||
|---|---|---|---|---|---|
| n | % | Mean [95% CI] | % | ||
| Baseline | 16 | 100 | 86.7 ± 23.3 | ||
| Week 1 | 14 | 87.5 | 88.3 ± 23.3 | − 0.5 [− 1.1, 0.1] | − 0.6 |
| Week 2 | 14 | 87.5 | 90.2 ± 21.0 | − 0.4 [− 1.1, 0.3] | − 0.4 |
| Week 3 | 13 | 81.3 | 87.3 ± 20.2 | − 1.0 [− 1.9, − 0.1] | − 1.2 |
| Week 4 | 13 | 81.3 | 87.2 ± 19.8 | − 1.0 [− 1.5, − 0.5] | − 1.1 |
| Week 5 | 10 | 62.5 | 80.9 ± 15.9 | − 1.2 [− 2.4, − 0.1] | − 1.3 |
| Week 6 | 13 | 81.3 | 86.2 ± 19.0 | − 2.0 ± [− 2.9, − 1.1] | − 2.2 |
| Follow-up (Week 7) | 12 | 75.0 | 85.0 ± 19.5 | − 2.7 ± [− 3.8, − 1.6] | − 2.9 |
Table 4.
Diet quality, daily mean ± SD
| Baseline (n = 13) | Follow-up (n = 12) | Change (mean [95% CI]) | |
|---|---|---|---|
| Calories, kcal | 1338 ± 443 | 1306 ± 496 | − 32 [− 338, 274] |
| Total fat, g | 55 ± 27 | 51 ± 26 | − 5 [− 19, 9] |
| Saturated fat, g | 18 ± 12 | 15 ± 10 | − 3 [− 9, 3] |
| Trans fat, g | 1 ± 1 | 1 ± 2 | 0 [− 1, 1] |
| Cholesterol, mg | 232 ± 125 | 258 ± 171 | 18 [− 49, 85] |
| Sodium, mg | 2225 ± 697 | 2056 ± 1405 | − 185 [− 986, 616] |
| Total carbohydrate, g | 151 ± 43 | 156 ± 51 | 4 [− 30, 38] |
| Dietary fiber, g | 18 ± 8 | 18 ± 6 | − 1 [− 5, 3] |
| Total sugars, g | 56 ± 21 | 58 ± 15 | − 3 [− 17, 11] |
| Added sugars | 25 ± 11 | 25 ± 13 | − 2 [− 12, 8] |
| Total protein, g | 63 ± 20 | 62 ± 26 | 1 [− 16, 18] |
| Vitamin D, mcg | 5 ± 5 | 6 ± 4 | 3 [0,6] |
| Calcium, mg | 643 ± 439 | 583 ± 273 | − 77 [− 287, 133] |
| Iron, mg | 14 ± 6 | 10 ± 4 | − 4 [− 8, 0] |
| Potassium, mg | 2093 ± 746 | 2281 ± 711 | 141 [− 307, 589] |
| Total fruits, servingsa | 1.6 ± 1.0 | 1.9 ± 1.2 | 0.2 [− 0.4, 0.8] |
| Whole fruits, servingsb | 1.4 ± 1.1 | 1.6 ± 1.3 | 0.1[− 0.5, 0.7] |
| Vegetables, servingsc | 3.1 ± 2.4 | 3.6 ± 1.9 | 0.3 [− 0.7, 1.3] |
None of the changes were statistically significant (all p > 0.05)
aTotal fruit: 100% citrus juice, 100% fruit juice excluding citrus juice, citrus fruit, fruit excluding citrus fruit, avocado and similar
bWhole fruit: citrus fruit, fruit excluding citrus fruit, avocado and similar
cVegetables: dark-green vegetables, deep-yellow vegetables, tomato, white potatoes, other starchy vegetables, legumes, other vegetables, 100% vegetable juice
Table 5.
Self-reported and objective physical activity, mean ± SD
| Minutes per week | Change | ||
|---|---|---|---|
| Baseline (n = 16) | Follow-up (n = 12) | ||
| Self-reported | |||
| Moderate-intensity exercise-related activities | 330 ± 301 | 395 ± 323 | 66 [− 178, 310] |
| All exercise-related activities | 1043 ± 526 | 1170 ± 458 | 127 [− 264, 518] |
| Week 3 (n = 11) | Week 6 (n = 9) | ||
| Physical activity tracker | |||
| Lightly active | 175 ± 57 | 168 ± 47 | − 10 [− 55, 35] |
| Fairly active | 20 ± 17 | 23 ± 17 | − 2 [− 13, 9] |
| Very active | 29 ± 23 | 30 ± 21 | − 3 [− 4, − 2] |
| Total activity | 224 ± 75 | 221 ± 56 | − 15 [− 76, 46] |
None of the changes were statistically significant (all p > 0.05)
Secondary measures
We were able to collect measurements of weight, dietary intake, and physical activity among participants who attended the intervention sessions. At follow-up, mean weight loss of participants was 2.7 ± 1.9 kg (2.9%) (Table 3). Imputing missing weight data did not substantively change weight change estimates (2.3 ± 1.9 kg or 2.4% weight loss after imputation).
There were no significant differences in self-reported energy, nutrients, or fruit and vegetable consumption (Table 4).
Similarly, although no significant changes in pre-post physical activity were reported (Table 5), participants completed 66 (95% CI − 178, 310) min more of moderate-intensity exercise-related activities from baseline to follow-up, and 127 (− 264, 518) min more of all exercise-related activities (Table 5). The Fitbits recorded greater intensity at follow-up, but overall minutes of activity did not increase from baseline to follow-up.
Discussion
To our knowledge, this is the first feasibility study testing telehealth delivery of the DPP in a senior center. We demonstrated feasibility through our ability to recruit the target sample and deliver the 6-week program. Program acceptability was high, with 75% of participants completing all six sessions, and valid self-reported diet, objective physical activity, and weight information obtained from the majority of participants. Secondary outcomes demonstrated a significant weight change of 2.9% at a rate of 0.2 to 0.5 kg per week over the 6-week intervention period. Suggestions for improvement included improving the sound quality of the connection between the health coach and participants, increasing the amount and depth of nutrition information presented, and adapting materials for older adults. Participants’ suggestions will be incorporated into the development of a larger scale study with sufficient power to assess the effectiveness of a telehealth adaptation of a year-long DPP intervention.
The seminal DPP trial demonstrated that lifestyle intervention reduced diabetes incidence by 58% (95% CI 48 to 66%) [13]; since then, the intervention has been implemented in hospital, community, workplace, and other settings [28]. In a study by West et al., the DPP program administered by lay health workers in senior centers resulted in a greater percentage of weight loss compared to control senior centers (38% of intervention group participants lost ≥ 5% of weight versus 5% among controls, p < 0.001) after 4 months [29]. The West et al. study had a high retention (83% attended at least 50% of sessions) [29] at an average cost of over $2731 per community center or $165 per participant [29, 30]. Medicare currently reimburses up to $690 per DPP participant achieving at least 5% weight loss and $190 for beneficiaries that do not meet the minimum weight loss goal [31]. However, 7 years after the West et al. study was completed, fewer than 10 of the 1452 nationwide DPP programs are based in senior centers.
Despite low penetrance of DPP programs, senior centers are well attended and remain a viable option. Congregate meals are enormously popular, with 95% of seniors in a nationwide survey indicating they would recommend them to a friend [32]. This highlights the importance of our feasibility findings and the opportunity for an increase of telehealth adaptations of the DPP in senior centers. Of the five senior centers we have partnered with in New York City, all but one have the resources available to conduct a telehealth adaptation of the DPP in their center.
There were several strengths of this feasibility study, including testing a proof of concept for an approach with broad scalability, fostering academic and community partnerships, and measuring quantitative and qualitative outcomes. Limitations of our study include a small sample size that was underpowered to detect changes in secondary outcomes and recruiting at one senior center already utilizing technology with older adults, who may be predisposed to have an interest and/or increased knowledge in technology use. Similar to other behavioral interventions that engage older adults, recruitment rates were very low [33, 34]. The 6-week intervention period was abbreviated from the year-long DPP intervention; however, the average number of DPP sessions attended in a large healthcare organization is six [35]. The space at this particular senior center was limited with frequent technology/connection difficulties, which may have impacted our ability to deliver the intervention. The low response rate from our initial outreach email suggests that alternative recruitment strategies would be necessary to achieve enrollment targets for a larger trial. At the recommendation of a senior center director, we are using recruitment strategies by telephone for our next study. Furthermore, the Diabetes Risk Screener was designed for physicians to identify people who may need testing for type 2 diabetes and not as a tool for referral to an intervention. As a result, three participants in the normal weight category met eligibility criteria due to age and other type 2 diabetes risk factors. Opportunities for improvement include environmental modification to accommodate a high prevalence of hearing loss that inhibits communication, tracking participants’ contributions to focus groups, so that changes in viewpoints could be assessed over time, allowing for greater flexibility for participants to return for follow-up appointments, and replacing the Diabetes Risk Score with blood glucose measures as the criteria for prediabetes. There were no significant differences in diet and physical activity measures despite significant weight loss, suggesting these measures may better serve as process, rather than outcome measures.
Conclusion
This study demonstrated the feasibility and acceptability for delivering an evidence-based intervention using telehealth among older adults. Results showed that telehealth adaptations are feasible for older adults and may be effective given the 2.9% weight loss at a rate of 0.2 to 0.5 kg per week over the 6-week intervention period. Moreover, this would meet the DPP goal of 5% weight loss if a larger program was sustained. Before evaluating a Medicare-reimbursable 12-month DPP intervention model, we are partnering with social support services such as the home-delivered meal program to investigate the feasibility of delivering a telehealth adaptation of the DPP within this population. This approach could provide an alternative strategy for disseminating information to improve health and reduce disease burden among aging populations.
Supplementary information
Additional file 1. Diabetes Prevention Program Group Lifestyle Balance™.
Additional file 2. CONSORT 2010 checklist of information to include when reporting a pilot or feasibility trial*.
Acknowledgements
The authors would like to thank study participants, the staff at Senior Planet, and Danielle Vargas for their contributions to this work.
Abbreviations
- BMI
Body mass index
- BRIDGE
BRInging the Diabetes prevention program to GEriatric populations
- CDC
Centers for Disease Control and Prevention
- CVD
Cardiovascular disease
- DPP
Diabetes Prevention Program
- NYC
New York City
- REDCap
Research Electronic Data Capture
- SD
Standard deviation
Authors’ contributions
JMB, JC, and JWR developed the study concept and design, interpreted the results, and wrote the initial manuscript. LK collected and analyzed the data. LK, JWR, MAS, LD, and JC commented on and edited the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by the New York Center for Diabetes Translational Research (P30DK111022-01). One of the co-authors (JWR) is a co-investigator for the New York Center for Diabetes Translational Research and provided mentorship to JMB on the design and conduct of the trial.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board at New York University Langone School of Medicine (i18-00229). All participants provided written informed consent.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jeannette M. Beasley, Phone: 646-501-4681, Email: jeannette.beasley@nyulangone.org
Lindsey Kirshner, Email: Lindsey.Kirshner@nyulangone.org.
Judith Wylie-Rosett, Email: Judith.Wylie-Rosett@einstein.yu.edu.
Mary Ann Sevick, Email: Mary.Sevick@nyulangone.org.
Laura DeLuca, Email: Ldeluca@mail.yu.edu.
Joshua Chodosh, Email: Joshua.Chodosh@nyulangone.org.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40814-019-0513-7.
References
- 1.United States Department of Commerce. US Census Bureau Quick Facts: Persons aged 65 and older: US Government; 2016 [July 20, 2017]. Available from: https://www.census.gov/quickfacts/fact/table/newyorkcountymanhattanboroughnewyork,newyorkcitynewyork, US/AGE775216.
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner EH. The role of patient care teams in chronic disease management. Bmj. 2000;320(7234):569–572. doi: 10.1136/bmj.320.7234.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010;3:CD005182. doi: 10.1002/14651858.CD005182.pub4. [DOI] [PubMed] [Google Scholar]
- 5.Margolis KL, Piller LB, Ford CE, Henriquez MA, Cushman WC, Einhorn PT, et al. Blood pressure control in Hispanics in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension (Dallas, Tex : 1979) 2007;50(5):854–861. doi: 10.1161/HYPERTENSIONAHA.107.092650. [DOI] [PubMed] [Google Scholar]
- 6.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289(16):2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 7.Boulware LE, Daumit GL, Frick KD, Minkovitz CS, Lawrence RS, Powe NR. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med. 2001;21(3):221–232. doi: 10.1016/S0749-3797(01)00356-7. [DOI] [PubMed] [Google Scholar]
- 8.Fahey T, Schroeder K, Ebrahim S. Educational and organisational interventions used to improve the management of hypertension in primary care: a systematic review. Brit J General Practice. 2005;55(520):875–882. [PMC free article] [PubMed] [Google Scholar]
- 9.Fahey T, Schroeder K, Ebrahim S. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Reviews. 2005;1:CD005182. doi: 10.1002/14651858.CD005182. [DOI] [PubMed] [Google Scholar]
- 10.Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Brit J General Practice. 2010;60(581):e476–e488. doi: 10.3399/bjgp10X544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munger MA, Van Tassell BW, LaFleur J. Medication nonadherence: an unrecognized cardiovascular risk factor. MedGenMed. 2007;9(3):58. [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Hypertension-related mortality among Hispanic subpopulations- United States, 1995-2002. Morbidity and Mortality Weekly Report (MMWR) 2006;55(7):177–180. [PubMed] [Google Scholar]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwan MW, Wong MC, Wang HH, Liu KQ, Lee CL, Yan BP, et al. Compliance with the dietary approaches to stop hypertension (DASH) diet: a systematic review. PLoS One. 2013;8(10):e78412. doi: 10.1371/journal.pone.0078412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 16.Kulick ER, Moon YP, Cheung K, Willey JZ, Sacco RL, Elkind MS. Racial-ethnic disparities in the association between risk factors and diabetes: the Northern Manhattan Study. Prev Med. 2016;83:31–36. doi: 10.1016/j.ypmed.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saydah SH, Imperatore G, Beckles GL. Socioeconomic status and mortality: contribution of health care access and psychological distress among U.S. adults with diagnosed diabetes. Diabetes Care. 2013;36(1):49–55. doi: 10.2337/dc11-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Medicare and Medicaid Services. Medicare Diabetes Prevention Program (MDPP) Expanded Model 2018. Available from: https://innovation.cms.gov/initiatives/medicare-diabetes-prevention-program/. Accessed 29 Oct 2019.
- 19.Scanlan AB, Maia CM, Perez A, Homko CJ, O'Brien MJ. Diabetes risk assessment in latinas: effectiveness of a brief diabetes risk questionnaire for detecting prediabetes in a community-based sample. Diabetes Spectrum. 2018;31(1):31–36. doi: 10.2337/ds16-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bang H, Edwards AM, Bomback AS, Ballantyne CM, Brillon D, Callahan MA, et al. Development and validation of a patient self-assessment score for diabetes risk. Ann Intern Med. 2009;151(11):775–783. doi: 10.7326/0003-4819-151-11-200912010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer MK, Vanderwood KK, Arena VC, Miller RG, Meehan R, Eaglehouse YL, et al. Evaluation of a diabetes prevention program lifestyle intervention in older adults: a randomized controlled study in three senior/community centers of varying socioeconomic status. Diabetes Educ. 2018;44(2):118–129. doi: 10.1177/0145721718759982. [DOI] [PubMed] [Google Scholar]
- 22.Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived alcohol use disorders identification test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcoholism. 2005;29(5):844–854. doi: 10.1097/01.ALC.0000164374.32229.A2. [DOI] [PubMed] [Google Scholar]
- 23.Tuunainen A, Langer RD, Klauber MR, Kripke DF. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res. 2001;103(2–3):261–270. doi: 10.1016/S0165-1781(01)00278-5. [DOI] [PubMed] [Google Scholar]
- 24.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 26.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aziz Z, Absetz P, Oldroyd J, Pronk NP, Oldenburg B. A systematic review of real-world diabetes prevention programs: learnings from the last 15 years. Implement Sci. 2015;10:172. doi: 10.1186/s13012-015-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West DS, Bursac Z, Cornell CE, Felix HC, Fausett JK, Krukowski RA, et al. Lay health educators translate a weight-loss intervention in senior centers: a randomized controlled trial. Am J Prev Med. 2011;41(4):385–391. doi: 10.1016/j.amepre.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krukowski RA, Pope RA, Love S, Lensing S, Felix HC, Prewitt TE, et al. Examination of costs for a lay health educator-delivered translation of the Diabetes Prevention Program in senior centers. Prev Med. 2013;57(4):400–402. doi: 10.1016/j.ypmed.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Medicare and Medicaid Services. Fact Sheet: Final Policies for the Medicare Diabetes Prevention Program Expanded Model in the Calendar Year 2018 Physician Fee Schedule Final Rule. Available from: https://innovation.cms.gov/Files/fact-sheet/mdpp-cy2018fr-fs.pdf. Accessed 29 Oct 2019.
- 32.Beasley JM, Sevick MA, Kirshner L, Mangold M, Chodosh J. Congregate meals: opportunities to help vulnerable older adults achieve diet and physical activity recommendations. J Frailty Aging. 2018;7(3):182–186. doi: 10.14283/jfa.2018.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copeland RJ, Horspool K, Humphreys L, Scott E, Booster trial t Recruiting to a large-scale physical activity randomised controlled trial - experiences with the gift of hindsight. Trials. 2016;17(1):104. doi: 10.1186/s13063-016-1229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatters R, Newbould L, Sprange K, Hind D, Mountain G, Shortland K, et al. Recruitment of older adults to three preventative lifestyle improvement studies. Trials. 2018;19(1):121. doi: 10.1186/s13063-018-2482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parsons AS, Raman V, Starr B, Zezza M, Rehm CD. Medicare underpayment for Diabetes Prevention Program: implications for DPP suppliers. Am J Manag Care. 2018;24(10):475–478. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Diabetes Prevention Program Group Lifestyle Balance™.
Additional file 2. CONSORT 2010 checklist of information to include when reporting a pilot or feasibility trial*.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.