Figure 1.
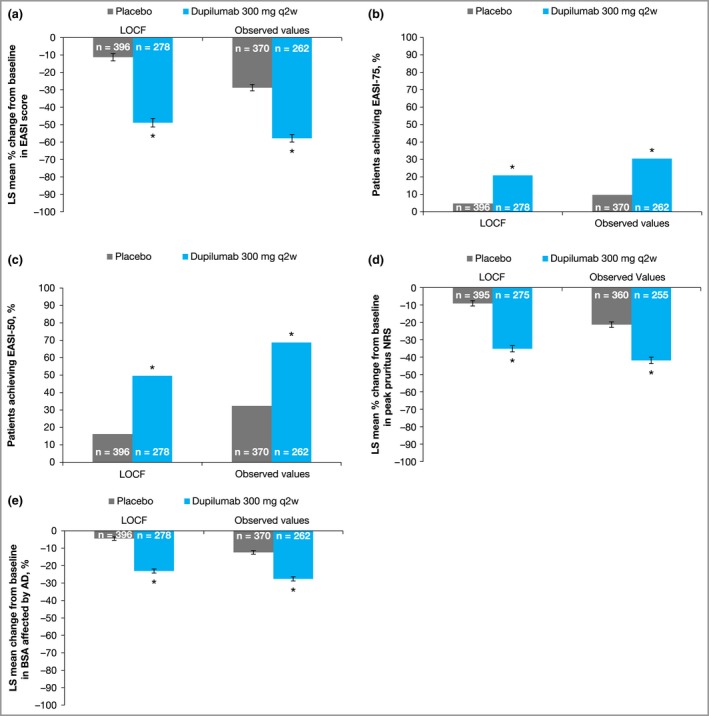
Outcomes in patients with Investigator's Global Assessment > 1 treated with dupilumab or placebo. (a) Percentage change from baseline to week 16 in Eczema Area and Severity Index (EASI) total score. (b, c) Proportions of patients who achieved ≥ 75% improvement in EASI (EASI 75) (b) or EASI 50 (c) at week 16. (d) Percentage change from baseline to week 16 in peak pruritus numerical rating scale (NRS) score. (e) Proportion of body surface area (BSA) affected by atopic dermatitis (AD). Error bars indicate standard error (SE). LOCF, last observation carried forward; LS, least squares; q2w, every 2 weeks. *P < 0·001 vs. placebo.
