Abstract
Objectives
To assess long‐term antiretroviral therapy (ART) outcomes during rapid HIV programme expansion in the public sector of Eswatini (formerly Swaziland).
Methods
This is a retrospectively established cohort of HIV‐positive adults (≥16 years) who started first‐line ART in 25 health facilities in Shiselweni (Eswatini) between 01/2006 and 12/2014. Temporal trends in ART attrition, treatment expansion and ART coverage were described over 9 years. We used flexible parametric survival models to assess the relationship between time to ART attrition and covariates.
Results
Of 24 772 ART initiations, 6% (n = 1488) occurred in 2006, vs. 13% (n = 3192) in 2014. Between these years, median CD4 cell count at ART initiation increased (113–265 cells/mm3). The active treatment cohort expanded 8.4‐fold, ART coverage increased 8.0‐fold (7.1% in 2006 vs. 56.8% in 2014) and 12‐month crude ART retention improved from 71% to 86%. Compared with the pre‐decentralisation period (2006–2007), attrition decreased by 5% (adjusted hazard ratio [aHR] 0.95, 95% confidence interval 0.88–1.02) during HIV‐TB service decentralisation (2008–2010), by 17% (aHR 0.83, 0.75–0.92) during service consolidation (2011–2012), and by 20% (aHR 0.80, 0.71–0.90) during further treatment expansion (2013–2014). The risk of attrition was higher for young age, male sex, pathological baseline haemoglobin and biochemistry results, more toxic drug regimens, WHO III/IV staging and low CD4 cell count; access to a telephone was protective.
Conclusions
Programmatic outcomes improved during large expansion of the treatment cohort and increased ART coverage. Changes in ART programming may have contributed to better outcomes.
Keywords: temporal trends, ART expansion, attrition, Swaziland, Eswatini
Abstract
Objectifs
Evaluer les résultats du traitement antirétroviral (ART) à long terme durant l'expansion rapide du programme de lutte contre le VIH dans le secteur public d'Eswatini (anciennement Swaziland).
Méthodes
Il s'agit d'une cohorte établie de manière rétrospective d'adultes VIH positifs (≥ 16 ans) qui ont commencé un ART de première ligne dans 25 établissements de santé à Shiselweni (Eswatini) entre janvier 2006 et décembre 2014. Les tendances temporelles de l'attrition dans l’ART, de l'extension de la couverture ART ont été décrites sur 9 ans. Nous avons utilisé des modèles de survie paramétriques flexibles pour évaluer la relation entre le temps écoulé avant l'attrition dans l’ART et les covariables.
Résultats
Sur 24.772 initiations à l’ART, 6% (n = 1.488) ont eu lieu en 2006 contre 13% (n = 3.192) en 2014. Entre ces années, le nombre médian de cellules CD4 au début du traitement ART a augmenté (113 à 265 cellules/mm3). La cohorte de traitement actif a été multipliée par 8,4, la couverture ART par 8,0 (7,1% en 2006 contre 56,8% en 2014) et la rétention brute sous ART est passée de 71% à 86%. Par rapport à la période antérieure à la décentralisation (2006‐2007), l'attrition a diminué de 5% (rapport de risque ajusté [aHR]: 0,95, intervalle de confiance à 95%: 0,88 à 1,02) au cours de la décentralisation des services VIH‐TB (2008‐2010), de 17% (HR: 0,83; 0,75‐0,92) lors de la consolidation du service (2011‐2012) et de 20% (HR: 0,80; 0,71‐0,90) lors de la poursuite de l'extension du traitement (2013‐2014). Le risque d'attrition était plus élevé pour le jeune âge, le sexe masculin, les résultats pathologiques de l'hémoglobine initiale et biochimiques, des schémas thérapeutiques plus toxiques, un stade III/IV de l’OMS et une faible numération des cellules CD4; l'accès au téléphone était protecteur.
Conclusions
Les résultats programmatiques se sont améliorés au cours de l'expansion importante de la cohorte de traitement et de l'augmentation de la couverture ART. Les changements apportés au programme ART peuvent avoir contribué à de meilleurs résultats.
Keywords: tendances temporelles, expansion de l’ART, attrition, Swaziland, Eswatini
Introduction
Because 4.1 million more people living with HIV (PLHIV) require HIV treatment for achievement of the second 90 of the UNAIDS 90‐90‐90 cascade targets in Eastern and Southern Africa 1, 2, effective and universal antiretroviral therapy (ART) provision remains the backbone of successful HIV programmes 2. WHO recommends a public health‐oriented approach for antiretroviral provision in resource‐limited settings (RLS), allowing for simplification of HIV services, service decentralisation and integration into tuberculosis (TB) and primary care settings, task‐shifting and expansion of treatment eligibility criteria 3, 4, 5, 6, 7. Despite the unprecedented progress in ART provision (e.g. 60% ART coverage in sub‐Saharan Africa in 2017) 1, 8, achievements remain fragile as challenges persist in funding, medical capacity, slow uptake and implementation of international recommendations, health service infrastructure, healthcare staffing, laboratory monitoring services, drug supply and programme monitoring 9, 10, 11, 12, 13, 14. Suboptimal ART retention has raised particular concern because large and rapidly expanding ART programmes appear to encounter greater challenges in retaining patients 15, 16. ART retention was 78% at 12 months in low‐ and middle‐income countries 15, and several studies found increased rates of loss to follow‐up (LTFU) in recent years 16, 17, 18, 19, 20, 21, 22, while mortality remained stable 17, 20 or decreased 17, 18, 21, 22. The mismatch of growing demand for ART and overburdened health facilities likely contributed to deterioration in quality of care, suboptimal adherence and treatment support and poorer record keeping 16, 17, 21. The feasibility of continued ART expansion – through the introduction of the WHO treat‐all approach endorsing prompt ART initiation irrespective of immunological status – remains uncertain and has resulted in doubts about its likely impact 10, 11, 12, 23, 24, 25, 26, 27, 28.
Eswatini (formerly Swaziland) has the highest HIV prevalence (32% in adults aged 18–49 years) 29 and one of the highest TB notification and HIV co‐infection rates in the world 30. Despite limited resources for the HIV response 31, 32, estimated HIV incidence decreased by 44% (from 2.5 to 1.4 cases per 100 person‐years) between 2011 and 2016 33. Population‐level viral load suppression among PLHIV was 73% in Eswatini and highest (79%) in the southern Shiselweni region 33, 34, surpassing the international UNAIDS population‐level target of 73% (1). Although the Eswatini ART response was considered a success story before the most recent guidance to drop all treatment eligibility criteria (treat‐all), contemporary large ART cohort analyses with extended patient follow‐up are lacking. We describe temporal trends in the growth and programmatic outcomes of the HIV treatment programme in the Shiselweni region of Eswatini, establishing a baseline against which to evaluate further treatment expansion, and drawing lessons to inform the implementation of universal treatment access.
Methods
Setting
The predominantly rural Shiselweni region (population 210 000 (35)) in southern Eswatini has an HIV prevalence of 26% in people aged ≥ 15 years 34. Several programmatic strategies were applied during ART expansion between 2006 and 2014 (Table 1). From 2006 to 2007, HIV‐TB care was centralised at outpatient departments of three secondary health facilities (two health centres, one hospital) in three health clusters (Nhlangano, Hlathikulu, Matsanjeni) (period‐1). In 2008, doctor‐led mobile outreach teams started decentralising HIV‐TB services, with physical integration into 22 nurse‐led primary care clinics from 2009 to 2010 (period‐2). Nurses were trained to perform ART and TB treatment initiation and follow‐up. Trained lay cadres provided HIV counselling and testing, basic laboratory tests (e.g. point‐of‐care CD4 cell count testing, biochemistry testing), medication dispensing, pre‐treatment counselling and treatment adherence support interventions. The WHO 2010 treatment guidelines were also endorsed in 2010. Thereafter, from 2011 to 2012, HIV services were consolidated, and community‐based HIV testing and routine viral load monitoring were introduced 36, 37 (period‐3). Then, in 2013 and 2014 (period‐4), ART eligibility was further extended in Nhlangano health cluster by phasing in the prevention of mother‐to‐child transmission (PMTCT) option B+ approach in 01/2013 38, 39 and universal ART provision irrespective of immunological criteria (treat‐all) in 10/2014. All services were provided free of charge by the Ministry of Health and Médecins Sans Frontières (MSF). A map of facilities of Shiselweni is presented in Figure S1:1.
Table 1.
Overview of main programmatic changes occurring during ART programme expansion (2006–2014)
| Programmatic periods | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐decentralisation (period‐1) | Decentralisationa (period‐2) | Consolidationb (period‐3) | Continued expansion (period‐4) | |||||||
| Decentralisation process | Centralised | Transition | Fully decentralised | |||||||
| ART eligibility | General PLHIV | ≤200 | ≤350c | |||||||
| PMTCT approach | Option A | Option A & option B+d | ||||||||
| Number of physically integrated HIV‐TB facilities | Primary care clinics | 0 | 0 | 0e | 4 | 21 | 22 | 22 | 22 | 22 |
| Secondary care facilities | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Viral load testing availability | No testing | Targeted testing | Routine monitoring | |||||||
| Expansion of community‐based HIV testing services | No | Yes | ||||||||
ART, antiretroviral therapy; PLHIV, people living with HIV; PMTCT, prevention of mother‐to‐child transmission.
Patients established on ART were also transferred down from secondary to primary care facilities.
Continued strengthening and streamlining of vertical integration of HIV and TB services as well as horizontal integration into routine primary care services.
One of the three health zones (Nhlangano health zone) provided the WHO treat‐all programmatic approach with ART initiation irrespective of CD4 and WHO staging criteria from October 2014.
PMTCT option B+ was provided in Nhlangano health zone from January 2013. Thereafter, it was phased in in the other two health zones from August 2014.
Mobile teams provided HIV‐TB care in a few facilities.
Study design and definitions
We analysed a retrospectively established cohort of HIV‐infected adults (≥16 years) initiated on standard first‐line ART in 25 health facilities in the Shiselweni region between 01/2006 and 12/2014. Data came from the national ART treatment database used for routine programmatic monitoring by the Ministry of Health. Exclusion criteria were transfer in from outside the region, missing age or being on a non‐standard first‐line treatment regimen. Follow‐up was from the day of ART initiation until the earliest of the composite outcome of all‐cause attrition (death or LTFU), transfer out of the region and database closure (08/2015). LTFU was defined as ≥ 6 months without a clinic visit, measured on the date of last clinic visit.
Statistics
First, we described patients’ baseline characteristics by calendar year and overall. Median and interquartile ranges (IQR) were used for continuous variables, and frequencies and proportions for categorical variables. Differences across categories were tested with Pearson's chi‐squared test.
Second, to estimate programmatic impact, we calculated the number of patients alive and retained in care at each calendar mid‐year, and obtained annual ART coverage estimates. The denominator was PLHIV, obtained by multiplying regional projected annual mid‐year population estimates 35 by regional HIV prevalence estimates for ≥15‐year‐olds 34. ART retention was calculated for each annual cohort separately using the Kaplan–Meier estimator.
Third, calendar years were grouped into corresponding programmatic periods (1–4) (Figure 1) for inclusion into covariate‐adjusted regression analysis, where time to attrition was the outcome. Other covariates for inclusion were determined a priori. We used multiple imputation by chained equations for imputation of missing covariate values of sex‐pregnancy status, CD4 cell count, WHO staging, body mass index (BMI), creatinine, alanine aminotransferase (ALT), haemoglobin and access to telephone, creating 20 imputed datasets 40. Then we used flexible parametric survival models (Royston–Parmar models) 41, 42 to describe the association between time to attrition and the covariates, and plotted averaged survival curves based on the fitted model 43. The number of internal knots for estimating the baseline spline function was based on Akaike's and Schwarz's Bayesian information criteria and on visual inspection of best fit of standardised survival and hazard curves. After assessment of the proportional hazards assumption with Schoenfeld residual statistics, we allowed the variable programmatic period to vary by duration on ART. Two regression models were built: the first included all available variables irrespective of magnitude of missingness (Model‐1), and the second was restricted to covariates with < 20% imputed values (Model‐2). In the sensitivity analysis, calendar year (instead of programmatic period) was allowed to interact with follow‐up time. All analyses were performed with Stata 14.1 (StataCorp, College Station, Texas, US).
Figure 1.
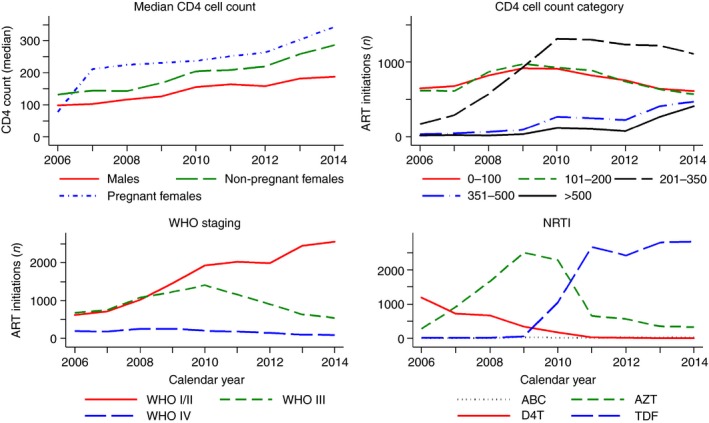
Temporal trends during ART programme expansion (2006–2014). ART, antiretroviral therapy; n, number; WHO, World Health Organization; NRTI, nucleoside reverse transcriptase inhibitor; AZT, zidovudine; ABC, abacavir; D4T, stavudine; TDF, tenofovir disoproxil fumarate. [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
Ethics
This study was approved by the Scientific and Ethics Committee of the Ministry of Health of Eswatini and the Health Sciences Faculty Research Ethics Committee of the University of Cape Town, South Africa. This research fulfilled the exemption criteria set by the MSF Ethics Review Board (ERB) for a posteriori analyses of routinely collected clinical data and thus did not require MSF ERB review. It was conducted with permission from Micaela Serafini (Medical Director, Operational Centre Geneva), MSF.
Results
Baseline characteristic and temporal trends
Baseline characteristics and temporal trends are presented in Tables 2, 3 and Figures 1, 2. Of 24 772 patients initiated on ART, Nhlangano health cluster contributed 10 451 (42%) observations. Annual initiations increased from 1488 in 2006 to 3536 in 2010, coinciding with HIV‐TB care decentralisation (2008–2010) and endorsement of the WHO 2010 treatment eligibility criteria. Thereafter, initiations decreased to 3039 in 2012, with a slight increase in 2013 and 2014 (n = 3188 and 3192), coinciding with the introduction of PMTCT option B+ and treat‐all.
Table 2.
Temporal trends in ART expansion and patient characteristics from 2006 to 2014. Values are numbers (%) or median (IQR)
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| ART initiations | 1488 | 1655 | 2354 | 2951 | 3536 | 3369 | 3039 | 3188 | 3192 | 24 772 |
| Men | 539 (36) | 580 (35) | 827 (35) | 1012 (34) | 1228 (35) | 1187 (35) | 1112 (37) | 1020 (32) | 988 (31) | 8493 (34) |
| Non‐pregnant women | 248 (17) | 462 (28) | 880 (37) | 1625 (55) | 1959 (55) | 1781 (53) | 1557 (51) | 1729 (54) | 1746 (55) | 11 987 (48) |
| Pregnant women | 3 (0) | 30 (2) | 86 (4) | 119 (4) | 172 (5) | 245 (7) | 268 (9) | 337 (11) | 383 (12) | 1643 (7) |
| Missinga | 698 (47) | 583 (35) | 561 (24) | 195 (7) | 177 (5) | 156 (5) | 102 (3) | 102 (3) | 75 (2) | 2649 (11) |
| Age, years (missing n = 0) | 35 (28–43.5) | 34 (29–43) | 34 (28–43) | 35 (28–45) | 34 (27–43) | 33 (27–42) | 33 (27–42) | 32 (26–41) | 31 (25–39) | 33 (27–42) |
| 16–24 | 166 (11) | 181 (11) | 302 (13) | 378 (13) | 504 (14) | 475 (14) | 451 (15) | 616 (19) | 686 (21) | 3759 (15) |
| 25–34 | 543 (36) | 665 (40) | 876 (37) | 1071 (36) | 1379 (39) | 1406 (42) | 1261 (41) | 1306 (41) | 1360 (43) | 9867 (40) |
| 35–44 | 439 (30) | 459 (28) | 645 (27) | 762 (26) | 879 (25) | 805 (24) | 712 (23) | 689 (22) | 654 (20) | 6044 (24) |
| 45–54 | 234 (16) | 212 (13) | 346 (15) | 449 (15) | 476 (13) | 437 (13) | 382 (13) | 364 (11) | 291 (9) | 3191 (13) |
| ≥55 | 106 (7) | 138 (8) | 185 (8) | 291 (10) | 298 (8) | 246 (7) | 233 (8) | 213 (7) | 201 (6) | 1911 (8) |
| CD4 count, cells/mm3 (missing n = 36) | 113 (55–174) | 127 (59–191) | 140 (70–213) | 157 (79–236) | 194 (98–289) | 198 (103–294) | 203 (100.5–295) | 243 (125–336) | 265 (136–368) | 183 (91‐287) |
| Men | 99 (42–157) | 102.5 (46–179) | 117 (53–187) | 127 (58–208) | 156 (73–256) | 164.5 (79–267.5) | 159 (69–259) | 182 (96–289) | 188 (87–297) | 145 (68‐245) |
| Non‐pregnant women | 132.5 (71.5–185) | 144.5 (66–200) | 143 (76–213) | 168 (92–246) | 205 (107–297) | 209 (117–299) | 220 (118.5–304) | 259 (143–345) | 287 (166–403) | 205 (108‐303) |
| Pregnant women | 78 (58–94) | 211.5 (146–291) | 225 (146–306) | 231 (153–279) | 237.5 (156.5–308.5) | 252 (176–319) | 264 (174–316) | 304 (220–432) | 343.5 (230–499) | 272 (186–346) |
| 0–100 | 651 (44) | 677 (41) | 824 (35) | 917 (31) | 911 (26) | 820 (24) | 759 (25) | 642 (20) | 616 (19) | 6817 (28) |
| 101–200 | 617 (42) | 616 (37) | 868 (37) | 976 (33) | 929 (26) | 890 (26) | 739 (24) | 638 (20) | 570 (18) | 6843 (28) |
| 201–350 | 170 (11) | 292 (18) | 576 (25) | 929 (32) | 1310 (37) | 1300 (39) | 1234 (41) | 1224 (38) | 1110 (35) | 8145 (33) |
| 351–500 | 32 (2) | 45 (3) | 66 (3) | 91 (3) | 268 (8) | 249 (7) | 226 (7) | 412 (13) | 473 (15) | 1862 (8) |
| ≥501 | 16 (1) | 23 (1) | 17 (1) | 33 (1) | 116 (3) | 106 (3) | 78 (3) | 268 (8) | 412 (13) | 1069 (4) |
| WHO staging (missing n = 13) | ||||||||||
| I+II | 615 (41) | 718 (43) | 1027 (44) | 1462 (50) | 1927 (55) | 2030 (60) | 1989 (65) | 2448 (77) | 2561 (80) | 14 777 (60) |
| III | 676 (45) | 754 (46) | 1077 (46) | 1230 (42) | 1407 (40) | 1159 (34) | 907 (30) | 637 (20) | 542 (17) | 8389 (34) |
| IV | 196 (13) | 183 (11) | 249 (11) | 258 (9) | 200 (6) | 177 (5) | 141 (5) | 101 (3) | 88 (3) | 1593 (6) |
| NRTI (missing n = 0) | ||||||||||
| ABC | 3 (0) | 2 (0) | 3 (0) | 39 (1) | 20 (1) | 15 (0) | 33 (1) | 27 (1) | 28 (1) | 170 (1) |
| AZT | 275 (18) | 917 (55) | 1664 (71) | 2503 (85) | 2292 (65) | 659 (20) | 564 (19) | 352 (11) | 330 (10) | 9556 (39) |
| D4T | 1192 (80) | 720 (44) | 664 (28) | 349 (12) | 181 (5) | 25 (1) | 20 (1) | 7 (0) | 9 (0) | 3167 (13) |
| TDF | 18 (1) | 16 (1) | 23 (1) | 60 (2) | 1043 (29) | 2670 (79) | 2422 (80) | 2802 (88) | 2825 (89) | 11 879 (48) |
| NNRTI (missing n = 0) | ||||||||||
| EFV | 475 (32) | 465 (28) | 506 (21) | 824 (28) | 1411 (40) | 2771 (82) | 2456 (81) | 2832 (89) | 2892 (91) | 14 632 (59) |
| NVP | 1013 (68) | 1190 (72) | 1848 (79) | 2127 (72) | 2125 (60) | 598 (18) | 583 (19) | 356 (11) | 300 (9) | 10 140 (41) |
n, number; IQR, interquartile range; WHO, World Health Organization; NRTI, nucleoside reverse transcriptase inhibitor; AZT, zidovudine; ABC, abacavir; D4T, stavudine; TDF, tenofovir disoproxil fumarate; NNRTI, non‐nucleoside reverse transcriptase inhibitors; EFV, efavirenz; NVP, nevirapine.
The greatest proportion of missing values was for pregnancy status (11%), while only <1% had missing sex status.
Table 3.
Baseline characteristics and predictors of all‐cause attrition, Model‐1
| (% missing values) | n (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR | 95% CI | aHR | 95% CI | ||
| Programmatic period; (0) | |||||
| Period‐1 | 3143 (12.7) | 1 | 1 | ||
| Period‐2 | 8841 (35.7) | 0.75 | (0.70–0.79) | 0.94 | (0.87–1.01) |
| Period‐3 | 6408 (25.9) | 0.57 | (0.53–0.61) | 0.83 | (0.75–0.92) |
| Period‐4 | 6380 (25.8) | 0.48 | (0.44–0.52) | 0.80 | (0.71–0.90) |
| Health cluster; (0) | |||||
| Nhlangano | 10 451 (42.2) | 1 | |||
| Hlathikulu | 8187 (33) | 1.02 | (0.97–1.08) | 1.04 | (0.98–1.10) |
| Matsanjeni | 6134 (24.8) | 1.05 | (0.99–1.11) | 0.95 | (0.89–1.02) |
| Sex‐pregnancy status; (10.7)a | |||||
| Non‐pregnant women | 11 987 (54.2) | 1 | 1 | ||
| Men | 8493 (38.4) | 1.22 | (1.16–1.28) | 1.23 | (1.15–1.32) |
| Pregnant women | 1643 (7.4) | 0.96 | (0.87–1.06) | 1.10 | (0.98–1.23) |
| Age, years; (0) | |||||
| 16–24 | 3759 (15.2) | 1.24 | (1.17–1.33) | 1.39 | (1.30–1.49) |
| 25–34 | 9867 (39.8) | 1 | 1 | ||
| 35–44 | 6044 (24.4) | 0.87 | (0.82–0.92) | 0.84 | (0.79–0.90) |
| 45–54 | 3191 (12.9) | 0.82 | (0.76–0.88) | 0.82 | (0.75–0.89) |
| ≥55 | 1911 (7.7) | 1.07 | (0.98–1.17) | 1.11 | (1.02–1.22) |
| CD4 cell count, cells/mm3; (0.1) | |||||
| 0–100 | 6817 (27.6) | 1.99 | (1.87–2.11) | 1.47 | (1.38–1.57) |
| 101–200 | 6843 (27.7) | 1.37 | (1.29–1.46) | 1.18 | (1.11–1.26) |
| 201–350 | 8145 (32.9) | 1 | 1 | ||
| 351–500 | 1862 (7.5) | 0.92 | (0.82–1.03) | 0.94 | (0.84–1.06) |
| ≥501 | 1069 (4.3) | 0.89 | (0.77–1.04) | 0.96 | (0.82–1.12) |
| WHO staging; (0.1) | |||||
| I+II | 14 777 (59.7) | 1 | 1 | ||
| III | 8389 (33.9) | 1.55 | (1.47–1.63) | 1.24 | (1.17–1.31) |
| IV | 1593 (6.4) | 3.03 | (2.82–3.27) | 2.06 | (1.88–2.25) |
| NRTI; (0) | |||||
| AZT | 9556 (38.6) | 1 | 1 | ||
| ABC | 170 (0.7) | 1.46 | (1.13–1.88) | 0.94 | (0.72–1.24) |
| D4T | 3167 (12.8) | 1.86 | (1.75–1.97) | 1.16 | (1.05–1.28) |
| TDF | 11 879 (48) | 0.82 | (0.78–0.87) | 0.93 | (0.84–1.02) |
| NNRTI; (0) | |||||
| EFV | 14 632 (59.1) | 1 | 1 | ||
| NVP | 10 140 (40.9) | 1.18 | (1.13–1.24) | 1.07 | (0.99–1.15) |
| BMI, kg/m2; (47.5) | |||||
| <18.5 | 1349 (10.4) | 1 | 1 | ||
| 18.5–24.9 | 7066 (54.3) | 0.74 | (0.69–0.81) | 0.98 | (0.90–1.07) |
| ≥25 | 4586 (35.3) | 0.54 | (0.49–0.60) | 0.93 | (0.82–1.06) |
| Creatinine, μmol/L; (59) | |||||
| ≤120 | 9630 (94.7) | 1 | 1 | ||
| 121–240 | 480 (4.7) | 1.16 | (0.86–1.57) | 0.86 | (0.63–1.17) |
| ≥241 | 55 (0.5) | 2.97 | (1.79–4.90) | 1.77 | (1.06–2.95) |
| ALT, U/L; (61.4) | |||||
| ≤42 | 8006 (83.8) | 1 | 1 | ||
| ≥43 | 1551 (16.2) | 1.19 | (1.09–1.31) | 1.12 | (1.01–1.25) |
| HB, mg/dL; (59.5) | |||||
| ≥10 | 8009 (79.9) | 1 | 1 | ||
| ≤9 | 2016 (20.1) | 2.11 | (1.98–2.26) | 1.65 | (1.50–1.81) |
| Access to telephoneb (33.3) | |||||
| No | 918 (5.6) | 1 | 1 | ||
| Yes | 15 613 (94.4) | 0.62 | (0.55–0.71) | 0.65 | (0.57–0.74) |
n, number; HR, hazard ratio; aHR, adjusted hazard ratio; CI, confidence interval; WHO, World Health Organization; NRTI, nucleoside reverse transcriptase inhibitor; AZT, zidovudine; ABC, abacavir; D4T, stavudine; TDF, tenofovir disoproxil fumarate; NNRTI, non‐nucleoside reverse transcriptase inhibitors; EFV, efavirenz; BMI, body mass index; NVP, nevirapine; ALT, alanine transaminase; HB, haemoglobin.
While 0.2% of patients had missing sex status, pregnancy status was missing in 16% of women.
Access to a telephone was defined as a patient with a recorded telephone number in the database.
Multiple imputations were used in all univariate and multivariate regression analyses for the variables sex‐pregnancy status, CD4 cell count, WHO staging, BMI, creatinine, ALT, HB and access to telephone. The model had 6 degrees of freedom (5 internal knots) for non‐time‐dependent covariates and 2 degrees of freedom (1 internal knot) for the time‐dependent covariate programmatic period.
Figure 2.
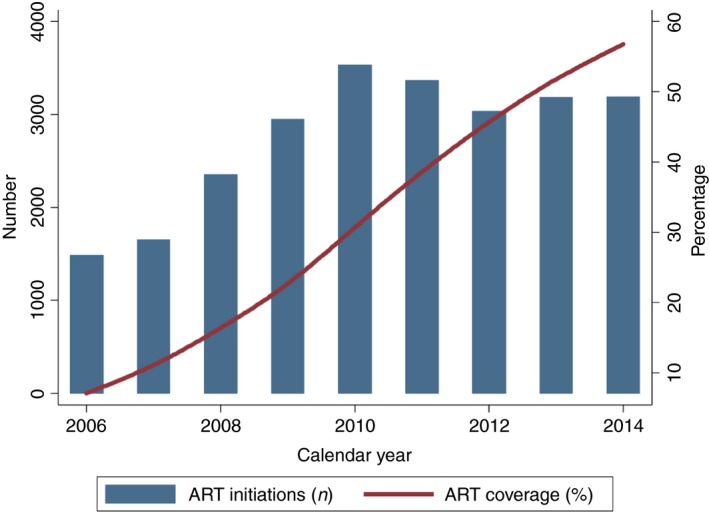
Annual number of ART initiations and treatment coverage during ART programme expansion (2014–2016). ART, antiretroviral therapy; n, number. [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
The median age at initiation was 33 (IQR 27–42) years with most patients being 25–34 (40%) and 35–44 (24%) years old. The proportion of young adults (16–24 years old) increased from 11% in 2006 to 21% in 2014, with a similar trend seen for 25‐ to 34‐year‐olds (2006: 36%; 2014: 43%). A reciprocal proportional decrease was seen for the age group 35–54 years, from 46% to 29%. The sex of 0.2% of patients was unknown and pregnancy status was missing in 16% of women. Most patients were non‐pregnant women (n = 11 987, 54%), followed by men (n = 8493, 38%) and pregnant women (n = 1643, 7%). Most patients (n = 15 613, 94%) had access to a telephone.
Most patients had CD4 cell counts of ≤100 (28%), 101–200 (28%) and 201–350 (33%) cells/mm3, while 12% had counts ≥351 cells/mm3. The median CD4 cell count increased in each consecutive year, most pronounced in pregnant women (from 78 to 343.5 cells/mm3), followed by non‐pregnant women (from 132.5 to 287 cells/mm3) and men (from 99 to 188 cells/mm3) (Figure 1). The absolute number of ART initiations at CD4 cell count ≤ 100 and 101–200 cells/mm3 increased until 2009 and decreased thereafter in consecutive years (Figure 1). 86% of patients had a CD4 cell count ≤ 200 cells/mm3 in 2006 vs. 37% in 2014. In the early years (2006–2009), ≤4% had CD4 cell counts ≥ 351 cells/mm3 vs. 28% in 2014. Overall, 8389 (34%) and 1593 (6%) of patients had WHO clinical stage III and IV, respectively, decreased from 45% and 13% in 2006 to 17% and 3% in 2014 (Figure 1).
The most widely used nucleoside reverse transcriptase inhibitor drugs were zidovudine (AZT) (39%) and tenofovir disoproxil fumarate (TDF) (48%). In 2006, 80% and 18% of patients received stavudine (D4T) and AZT compared with 89% and 10% receiving TDF and AZT in 2014 (Figure 1). D4T was largely phased out between 2009 and 2010, while TDF was phased in. Abacavir (ABC) was rarely used in all calendar years (≤1%). In 2006, the main non‐nucleoside reverse transcriptase inhibitor drug was nevirapine (NVP) (68%), while efavirenz (EFV) use increased rapidly from 2011, reaching 91% in 2014.
Laboratory measures for creatinine, ALT and haemoglobin were frequently not available at baseline (60%). Among patients with complete observations (Table 3), 1349 (10%) had a BMI < 18.5 kg/m2, 535 (5%) elevated creatinine ≥ 121 μmol/L, 1551 (16%) elevated ALT ≥ 43 U/L and 2016 (20%) anaemia (haemoglobin ≤ 9 mg/dL).
Programmatic impact
The number of people active on ART increased in consecutive years from 2171 in 2006 to 18 307 in 2014 (8.4‐fold increase). While the relative increase was similar for both sexes (women 8.5‐ vs. men 8.3‐fold increase), more women (n = 12 210, 67%) were on ART in 2014. In 2014, 9782 (53%) patients were followed at primary care level and 5477 (30%) at secondary care level, and 3048 (17%) did not have the healthcare level recorded. The ART coverage among PLHIV increased 8.0‐fold, from 7.1% in 2006 to 56.8% in 2014 (Figure 2).
Crude ART retention increased in consecutive annual ART cohorts (Figure 3). The 12‐month retention was 71% in 2006 compared with 86% in 2014. After 9 years of follow‐up of the 2006 ART cohort, 49% patients were retained in care. Improvements in retention were most pronounced comparing programmatic periods (Figure 3): retention was 66%, 74%, 80% and 82% at 2 years for consecutive periods 1–4.
Figure 3.
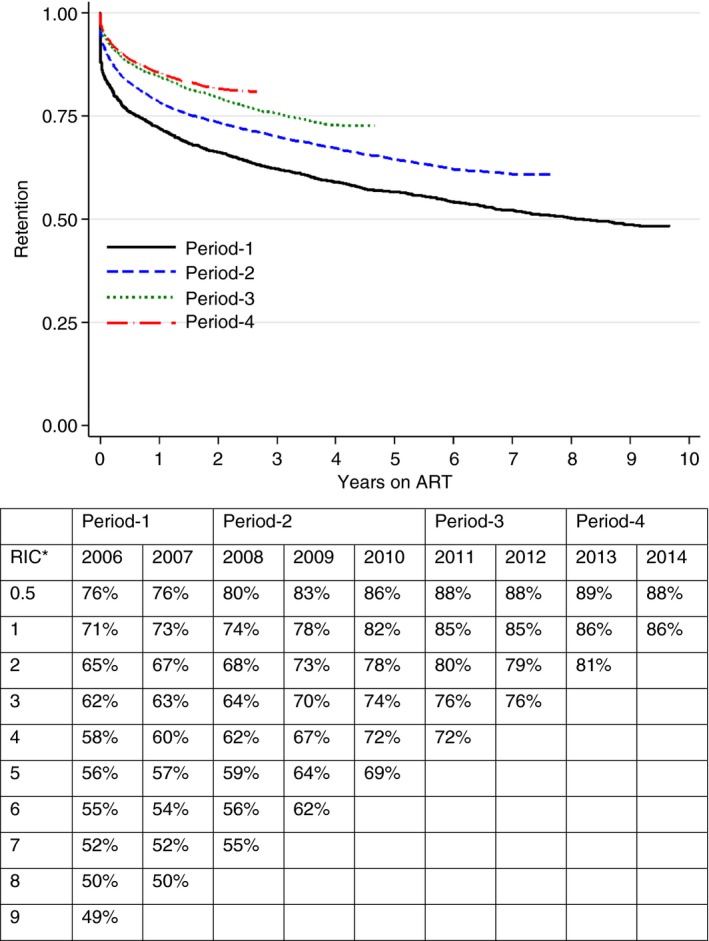
Crude ART retention by annual and programmatic periods. *RIC, retention in care in years; ART, antiretroviral therapy. [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
Associations with attrition
The total and median follow‐up times were 77 009.8 and 2.6 (IQR 1.0–4.9) years, with 1666 (7%) deaths and 5681 (23%) LTFU recorded at a rate of 9.5 (95% confidence interval [CI] 9.3–9.8) per 100 person‐years. Imputation diagnostic and model selection procedures are presented in Appendices S2–S3.
While only findings of Model‐1 are described here (Table 3), estimates were comparable between the models (Appendix S4, Table S4:1). Compared with the pre‐decentralisation period (2006–2007, period‐1), the overall risk of attrition dropped by 5% on average (adjusted hazard ratio [aHR] 0.95, 95% CI 0.88–1.02) during HIV‐TB service decentralisation (2008–2010, period‐2), by 17% (aHR 0.83, 0.75–0.92) during service consolidation (2011–2012, period‐3) and by 20% (aHR 0.80, 0.71–0.90) during further treatment expansion (2013–2014, period‐4). The effect, however, varied by duration on ART, with an absolute survival benefit seen during the first 2.5 (period‐2), 4.5 (period‐3) and 6.5 (period‐4) years following ART initiation (Figure 4b–d). Thereafter, it remained similar for period‐4 (compared with the pre‐decentralisation period) while tending to decrease for period‐2 and period‐3. Sensitivity analysis by calendar year rather than period showed increased risk of attrition during 2007 to 2008, similar risk in 2009 and decreasing attrition thereafter (Appendix S4, Figure S4:1).
Figure 4.
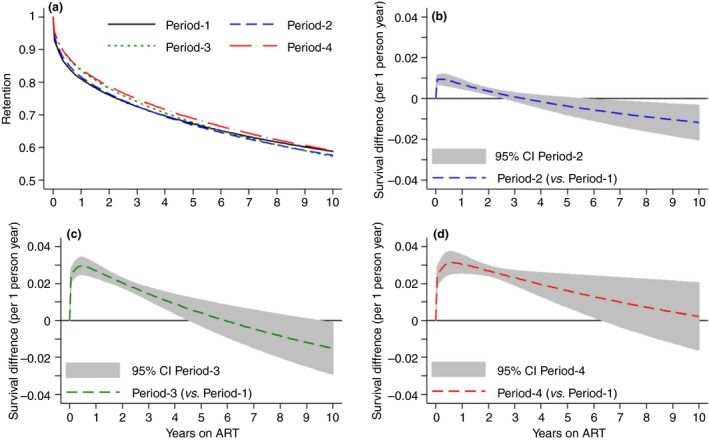
Standardised (average) survival curves (a) and survival difference (b–d) curves with 95% confidence interval by programmatic period. The overall 5% (aHR 0.95, 95% CI 0.88–1.02), 17% (aHR 0.83, 0.75–0.92) and 20% (aHR 0.80, 0.71–0.90) decreased risk of attrition varied by time on ART for periods 2–4 compared with period‐1. An absolute survival benefit was seen during 2.5 (period‐2), 4.5 (period‐3) and 6.5 (period‐4) years following ART initiation. Thereafter, it remained similar for period‐4 (compared with the pre‐decentralisation period), while it tended to decrease for period‐2 and period‐3. aHR, adjusted hazard ratio; CI, confidence interval. [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
Compared with non‐pregnant women, men had a 23% (aHR 1.23, 1.15–1.32) increased risk of attrition while pregnant women had a 10% increased risk (aHR 1.10, 0.98–1.23) (Appendix S4, Figure S4:2a). Compared with the reference age group 25–34 years, attrition was increased for young adults (16–24 years) and patients aged ≥ 55 years, while it was decreased for patients aged 35–54 years. The availability of a phone decreased attrition by 35% (aHR 0.65, 0.57–0.74) while BMI and health cluster did not show associations.
Compared with CD4 cell count 201–350 cells/mm3, lower CD4 strata had a higher risk of attrition (CD4 ≤ 100: aHR 1.47, 1.38–1.57; CD4 101–200: aHR 1.18, 1.11–1.26), while higher CD4 cell counts had similar outcomes (Appendix S4, Figure S4:2b). Patients with WHO III and IV staging also had an increased risk (WHO III: aHR 1.24, 1.17–1.31; WHO IV: aHR 2.06, 1.88–2.25), as did patients with elevated values of creatinine or ALT, or anaemia.
While Model‐2 (Appendix S4, Table S4:1) showed increased risk of attrition for ABC (aHR 1.34, 95% CI 1.04–1.73) and D4T (aHR 1.57, 1.47–1.69) compared with AZT, Model‐1 showed an increased risk only for D4T (aHR 1.16, 1.05–1.28).
Discussion
This study describes temporal trends and outcomes of public sector ART programme expansion in a predominantly rural setting over 9 years. Crude attrition decreased over time coinciding with an 8‐fold expansion of the treatment cohort and an increase in ART coverage. The same trend was seen in covariate‐adjusted analysis of attrition, coinciding with programmatic changes over time.
Results in context
Crude ART retention of the 2010 annual cohort and thereafter compares favourably with retention reported from routine low‐ and middle‐income settings (78% at 12 months, 71% at 24 months and 69% at 36 months) 15. In contrast to other studies suggesting higher attrition for more recent cohorts 15, 21, attrition decreased with each consecutive programmatic period and was reduced by 20% in 2012–2014. Firstly, one study from neighbouring South Africa ceased patient follow‐up during the early years of programme expansion 21, possibly missing positive trends once HIV‐TB programmes had been consolidated. Secondly, the implementation of combination interventions – ART provision at higher CD4 thresholds, prescription of less toxic drug combinations – may have supported ART expansion while maintaining and improving outcomes. Active defaulter tracing by phone (with possibility of physical tracing by home visits) may also have contributed, as patients with access to a phone had a 35% decreased risk of attrition. Other studies showed that programmes applying physical defaulter tracing had higher 12‐month retention on ART (80.0% vs. 75.8%) 44 and programmes using phones in combination interventions had reduced loss to care 44, 45, 46, 47. Sensitivity analysis, however, suggested a temporary increase in risk of attrition in 2008, the year when HIV‐TB care was decentralised through mobile doctor‐led teams. The rapid introduction of new programmatic and clinical activities (decentralisation, task‐shifting and sharing, provision of ART and TB treatment in previous ART‐TB ‘naïve’ primary care clinics) facilitated access to ART but may have overwhelmed the health system and health workers. Although attrition showed a tendency of decline since 2010 (the end of the decentralisation period), the full gains became evident later during the consolidation period in 2012, when risk of attrition was reduced by 17%.
Similar to other studies, young age 17, 32, 48, 49, 50, 51, 52, 53, sex‐pregnancy status 17, 32, 48, 49, 51, 54, 55, 56, pathological baseline laboratory test results 48, more toxic drug regimens (e.g. D4T) 53, advanced WHO III/IV staging 32, 48, 50, 51, 52, 53, 56 and low CD4 cell counts 17, 48, 49, 50, 52 increased the risk of an adverse outcome. Notably, patients with CD4 cell counts above 350 cells/mm3 had similar outcomes to patients with CD4 cell count 201–350 cells/mm3, suggesting that expansion of ART eligibility through the WHO treat‐all approach is feasible for healthier individuals.
Explanation of results and programmatic lessons learnt
Service decentralisation and integration, task‐shifting, simplification of HIV care, viral load testing and innovative care models were found efficient to support ART expansion in RLS 7, 51, 52, 53, 54, 57, 58, 59. In this setting, timely identification of programmatic obstacles and overcoming them with effective combination interventions were likely contributing factors for improved programmatic outcomes.
First, the highly centralised set‐up of HIV‐TB care provision required decentralisation and integration of services into rural primary care clinics to increase access and facilitate the programmatic and clinical management of HIV‐TB co‐infection. Second, the human resources for health crisis intertwined with lack of patient support structures necessitated task‐shifting and sharing. Nurses were capacitated to diagnose and treat HIV‐TB with only limited doctor support. Lay people living with HIV from the surrounding communities were trained to provide basic clinic services and patient support. Third, to increase access to timely treatment, international policy changes were quickly implemented (e.g. 2010 WHO treatment eligibility recommendations) and pilot interventions for treatment expansion were introduced before becoming WHO policy (e.g. phase‐in of PMTCT option B+ 38, 39 and treat‐all). This allowed an increasing number of PLHIV to start treatment before presenting with advanced HIV disease, possibly resulting in a less sick and clinically more easy to manage treatment cohort. In this study, however, only a limited number of patients were exposed to the most recent policy changes (PMTCT option B+, treat‐all), thus making it difficult to analyse the possible impact of these changes on patient outcomes. Fourth, the establishment of phlebotomist‐led mini‐laboratories at primary care clinics allowed for point‐of‐care CD4, haemoglobin and biochemistry (creatinine, ALT) testing 60. This was likely to support rapid ART initiation and timely clinical decision‐making by reducing turnaround time between sample collection and result delivery. Fifth, the introduction of routine viral load monitoring and enhanced adherence counselling for patients with elevated viral loads 37, 61 was likely to reduce mortality, LTFU and development of drug resistance 62, 63.
Although the findings of this study can be considered favourable, other programmatic constraints such as suboptimal linkage to care from routine community‐based (34%) and facility‐based (87%) HIV testing 36, 45 may jeopardise achievement of the UNAIDS 90‐90‐90 targets. However, this setting reported favourable viral load outcomes, with 84% of ART patients having an undetectable viral load (<100 copies/mL) 61 and 89% of pregnant and lactating women achieving viral suppression (<1000 copies/mL) 39. Future programmatic changes such as simplification of ART provision under the treat‐all approach and the introduction of potent antiretroviral drugs (e.g. dolutegravir) could have the potential to further improve outcomes. The 2017 WHO recommendation on rapid and same‐day ART initiation is also expected to improve rates of treatment initiation, time to viral suppression and treatment outcomes 64, 65.
Strengths and weaknesses
Although the introduction of specific programmatic and health policy factors coincided with improved outcomes over time, this study was not designed to assess causal relationships. The efficacy and real‐world effectiveness of the intervention in routine settings were discussed previously 66, 67, 68. Bias could have been introduced by not adjusting for other undocumented factors possibly associated with the outcome (e.g. TB co‐infection, socioeconomic and facility‐level factors). Treatment interruptions were not taken into account as longitudinal visit data were not available. This may have overestimated LTFU for more recent cohorts with shorter follow‐up time 69. Exclusion of treatment interrupters re‐entering ART care may underestimate programmatic impact if the reason for return was a defaulter tracing intervention integrated into the ART programme. Because ascertainment of patient outcomes was weak and we had no access to national death registries, LTFU and death were combined into the composite outcome of all‐cause attrition. Thus, we were likely to miss separate temporal trends in mortality and LTFU as reported from other settings 16, 17, 18, 19, 20, 21, 22. It was beyond the scope of this study to assess HIV testing, linkage to HIV care and viral load suppression, all factors crucial to estimating the overall success of large HIV programmes. Finally, despite the overall success of this HIV treatment programme, outcomes of children remained unknown as the analysis was limited to young people and adults.
A strength of the study was the use of international recommendations for definitions of LTFU and methodological approaches to ART survival analysis 70, 71. In addition, flexible parametric survival methods allowed us to easily model programmatic period as a time‐dependent factor and to understand its impact on survival over time. As this study originated from the public sector ART programme, findings are likely generalisable to other similar rural and resource‐poor settings in sub‐Saharan Africa.
Conclusions
Documenting and measuring previous progress in ART programming provides lessons for future treatment expansion. In this setting, public sector ART expansion was feasible and yielded improved programmatic outcomes over time. HIV‐TB service decentralisation and integration, task‐shifting and progressive changes in treatment eligibility were likely key factors facilitating access to ART and increasing treatment coverage. After years of ART programming, this setting appears prepared for continued treatment expansion through the WHO treat‐all approach.
Supporting information
Appendix S1. Setting.
Appendix S2. Diagnostic after multiple imputation.
Appendix S3. Building the flexible parametric survival model.
Appendix S4. Results.
Acknowledgements
We thank all the patients and health care workers who supported the scale‐up of HIV and TB care in the Shiselweni region.
References
- 1. UNAIDS data 2017 [Internet]. Joint United Nations Programme on HIV/AIDS (UNAIDS): Geneva, Switzerland; 2017. Available from: http://www.unaids.org/en/resources/documents/2017/2017_data_book [Google Scholar]
- 2. 90‐90‐90 An ambitious treatment target to help end the AIDS epidemic. UNAIDS: Geneva, Switzerland; 2014. [Google Scholar]
- 3. Hirnschall G, Harries AD, Easterbrook PJ, Doherty MC, Ball A. The next generation of the World Health Organization's global antiretroviral guidance. J Int AIDS Soc 2013: 16: 18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization , Gilks C, Vitória M. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. World Health Organization: Geneva, Switzerland; 2006. (Available from: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf) [6 Jan 2017]. [Google Scholar]
- 5. World Health Organization . Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. World Health Organization: Geneva, Switzerland; 2013. (Available from: http://www.ncbi.nlm.nih.gov/books/NBK195400/) [1 Dec 2014] [PubMed] [Google Scholar]
- 6. World Health Organization, Department of HIV/AIDS . Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. World Health Organization: Geneva, Switzerland; 2016. (Available from: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf) [31 Jul 2016]. [Google Scholar]
- 7. World Health Organization . Operations Manual for Delivery of HIV Prevention, Care, and Treatment at Primary Health Centres in High‐prevalence, Resource‐constrained Settings. World Health Organization: Geneva, Switzerland; 2008. (Available from: http://www.ncbi.nlm.nih.gov/books/NBK310908/) [9 Jan 2017]. [PubMed] [Google Scholar]
- 8. Harries AD, Suthar AB, Takarinda KC et al Ending the HIV/AIDS epidemic in low‐ and middle‐income countries by 2030: is it possible? F1000Research 2016; 5: 2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takarinda KC, Harries AD, Mutasa‐Apollo T. Critical considerations for adopting the HIV “treat all” approach in Zimbabwe: is the nation poised? Public Health Action 2016: 6: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rutherford GW, Anglemyer A. Is 90‐90‐90 achievable? Lancet HIV 2017: 4: 193–194. [DOI] [PubMed] [Google Scholar]
- 11. Vella S. End of AIDS on the horizon, but innovation needed to end HIV. Lancet HIV 2015: 2: e74–e75. [DOI] [PubMed] [Google Scholar]
- 12. Vella S. Addressing barriers to the end of AIDS by 2030. Lancet HIV 2015: 2: e360–e361. [DOI] [PubMed] [Google Scholar]
- 13. Celletti F, Cohn J, Connor C, Lee S, Giphart A, Montaner J. From policy to action: how to operationalize the treatment for all agenda. J Int AIDS Soc 2016: 19: 21185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bekker L‐G, Venter F, Cohen K et al Provision of antiretroviral therapy in South Africa: the nuts and bolts. Antivir Ther 2014: 19(Suppl 3): 105–116. [DOI] [PubMed] [Google Scholar]
- 15. Fox MP, Rosen S. Retention of adult patients on antiretroviral therapy in low‐ and middle‐income countries: systematic review and meta‐analysis 2008–2013. J Acquir Immune Defic Syndr 1999 2015:69:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grimsrud A, Balkan S, Casas EC et al Outcomes of antiretroviral therapy over a 10‐year period of expansion: a multicohort analysis of African and Asian HIV programs. J Acquir Immune Defic Syndr 1999 2014:67:e55–e66. [DOI] [PubMed] [Google Scholar]
- 17. Nglazi MD, Lawn SD, Kaplan R et al Changes in programmatic outcomes during 7 years of scale‐up at a community‐based antiretroviral treatment service in South Africa. JAIDS J Acquir Immune Defic Syndr 2011: 56: e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Auld AF, Shiraishi RW, Couto A et al A decade of antiretroviral therapy scale‐up in Mozambique: Evaluation of outcome trends and new models of service delivery among more than 300,000 patients enrolled during 2004‐2013. J Acquir Immune Defic Syndr 1999 2016: 73: e11–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mutevedzi PC, Lessells RJ, Newell M‐L. Disengagement from care in a decentralised primary health care antiretroviral treatment programme: cohort study in rural South Africa. Trop Med Int Health 2013: 18: 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Auld AF, Ekra KA, Shiraishi RW et al Temporal trends in treatment outcomes for HIV‐1 and HIV‐2‐infected adults enrolled in Côte d'Ivoire's national antiretroviral therapy program. PLoS ONE 2014: 9: e98183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cornell M, Grimsrud A, Fairall L et al Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS Lond Engl 2010: 24: 2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boulle A, Van Cutsem G, Hilderbrand K et al Seven‐year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS 2010: 24: 563–572. [DOI] [PubMed] [Google Scholar]
- 23. Stover J, Bollinger L, Izazola JA, Loures L, DeLay P, Ghys PD. What is required to end the AIDS epidemic as a public health threat by 2030? The cost and impact of the Fast‐Track approach. PLoS ONE 2016: 11: e0158253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eholié SP, Badje A, Kouame GM et al Antiretroviral treatment regardless of CD4 count: the universal answer to a contextual question. AIDS Res Ther 2016: 13: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olney JJ, Braitstein P, Eaton JW et al Evaluating strategies to improve HIV care outcomes in Kenya: a modelling study. Lancet HIV 2016: 3: e592–e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilson DP, Stoové MA, Hellard M. A reality check for aspirational targets to end HIV. Lancet HIV 2015: 2: e11. [DOI] [PubMed] [Google Scholar]
- 27. Fox MP. Are we shifting attrition downstream in the HIV cascade? Lancet HIV 2016: 3: e554–e555. [DOI] [PubMed] [Google Scholar]
- 28. Abdool Karim SS. Overcoming impediments to global implementation of early antiretroviral therapy. N Engl J Med 2015: 373: 875–876. [DOI] [PubMed] [Google Scholar]
- 29. Bicego GT, Nkambule R, Peterson I et al Recent patterns in population‐based HIV prevalence in Swaziland. PLoS ONE 2013: 8: e77101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Global tuberculosis report. World Health Organization: Geneva, Switzerland, 2017, 2017. [Google Scholar]
- 31. Auld AF, Kamiru H, Azih C et al Implementation and operational research: evaluation of Swaziland's Hub‐and‐Spoke model for decentralizing access to antiretroviral therapy services. J Acquir Immune Defic Syndr 1999 2015: 69: e1–e12. [DOI] [PubMed] [Google Scholar]
- 32. Khumalo PG, Chou YJ, Pu C. Antiretroviral treatment attrition in Swaziland: a population‐based study. Epidemiol Infect 2016: 44: 3474–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nkambule R, Nuwagaba‐Biribonwoha H, Mnisi Z et al Substantial progress in confronting the HIV epidemic in Swaziland‐ First evidence of national impact. In Paris, France; 2017. (Available from: http://programme.ias2017.org/Abstract/Abstract/5837)
- 34. Swaziland HIV incidence measurement survey 2: Population‐based HIV impact assessment, SHIMS2 2016‐2017; Summary sheet preliminary findings. Ministry of Health Swaziland: Mbabane, Swaziland; 2017. [Google Scholar]
- 35. 2007 Swaziland Population Census. Central Statistical Office (CSO): Mbabane, Swaziland, 2009. [Google Scholar]
- 36. Parker LA, Jobanputra K, Rusike L et al Feasibility and effectiveness of two community‐based HIV testing models in rural Swaziland. Trop Med Int Health 2015: 20: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jobanputra K, Parker LA, Azih C et al Impact and programmatic implications of routine viral load monitoring in Swaziland. J Acquir Immune Defic Syndr 1999 2014: 67:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parker LA, Jobanputra K, Okello V et al Barriers and facilitators to combined ART initiation in pregnant women with HIV: lessons learnt from a PMTCT B+ pilot program in Swaziland. JAIDS J Acquir Immune Defic Syndr 2015: 69: e24–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Etoori D, Kerschberger B, Staderini N, Ndlangamandla M, Nhlabatsi B, Jobanputra K et al Challenges and successes in the implementation of option B+ to prevent mother‐to‐child transmission of HIV in southern Swaziland. BMC Public Health 2018: 18: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011: 30: 377–399. [DOI] [PubMed] [Google Scholar]
- 41. Royston P, Lambert PC. Flexible Parametric Survival Analysis Using Stata: Beyond the Cox Model. StataCorp LP: Texas, US, 2011. [Google Scholar]
- 42. Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J 2009: 9: 265–290. [Google Scholar]
- 43. Lambert P. STPM2_STANDSURV: Stata module to obtain standardized survival curves after fitting an stpm2 survival model. Stat Softw Compon, 2017. (Available from: https://ideas.repec.org/c/boc/bocode/s458289.html) [1 Apr 2018].
- 44. McMahon JH, Elliott JH, Hong SY, Bertagnolio S, Jordan MR. Effects of physical tracing on estimates of loss to follow‐up, mortality and retention in low and middle income country antiretroviral therapy programs: a systematic review. PLoS ONE 2013: 8: e56047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McNairy ML, Lamb MR, Gachuhi AB et al Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: the Link4Health cluster randomized trial. PLoS Med 2017: 14: e1002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mehta K, Kumar AMV, Chawla S et al “M‐TRACK” (mobile phone reminders and electronic tracking tool) cuts the risk of pre‐treatment loss to follow‐up by 80% among people living with HIV under programme settings: a mixed‐methods study from Gujarat, India. Glob Health Action 2018: 11: 1438239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Izudi J, Mugenyi J, Mugabekazi M et al Retention of HIV‐positive adolescents in care: a quality improvement intervention in mid‐western Uganda. Biomed Res Int 2018: 2018: 1524016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moyo F, Chasela C, Brennan AT et al Treatment outcomes of HIV‐positive patients on first‐line antiretroviral therapy in private versus public HIV clinics in Johannesburg, South Africa. Clin Epidemiol 2016: 8: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hassan AS, Mwaringa SM, Ndirangu KK, Sanders EJ, de Wit TFR, Berkley JA. Incidence and predictors of attrition from antiretroviral care among adults in a rural HIV clinic in Coastal Kenya: a retrospective cohort study. BMC Public Health 2015: 15: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meloni ST, Chang C, Chaplin B et al Time‐dependent predictors of loss to follow‐up in a large HIV treatment cohort in Nigeria. Open Forum Infect Dis 2014; 1: ofu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koole O, Houben RM, Mzembe T et al Improved retention of patients starting antiretroviral treatment in Karonga District, northern Malawi, 2005‐2012. J Acquir Immune Defic Syndr 1999 2014: 67: e27–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koole O, Tsui S, Wabwire‐Mangen F et al Retention and risk factors for attrition among adults in antiretroviral treatment programmes in Tanzania, Uganda and Zambia. Trop Med Int Health 2014: 19: 1397–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Odafe S, Torpey K, Khamofu H et al The pattern of attrition from an antiretroviral treatment program in Nigeria. PLoS ONE 2012: 7: e51254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boyles TH, Wilkinson LS, Leisegang R, Maartens G. Factors influencing retention in care after starting antiretroviral therapy in a rural South African programme. PLoS ONE 2011: 6: e19201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang B, Losina E, Stark R et al Loss to follow‐up in a community clinic in South Africa – Roles of gender, pregnancy and CD4 count. South Afr Med 2011: 101: 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Auld AF, Mbofana F, Shiraishi RW et al Four‐year treatment outcomes of adult patients enrolled in Mozambique's rapidly expanding antiretroviral therapy program. PLoS ONE 2011: 6: e18453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lynch S, Ford N, van Cutsem G et al Getting HIV treatment to the most people. Science 2012: 337: 298–300. [DOI] [PubMed] [Google Scholar]
- 58. Suthar AB, Rutherford GW, Horvath T, Doherty MC, Negussie EK. Improving antiretroviral therapy scale‐up and effectiveness through service integration and decentralization. AIDS Lond Engl 2014: 28(Suppl 2): S175–S185. [DOI] [PubMed] [Google Scholar]
- 59. Mdege ND, Chindove S, Ali S. The effectiveness and cost implications of task‐shifting in the delivery of antiretroviral therapy to HIV‐infected patients: a systematic review. Health Policy Plan 2013: 28: 223–236. [DOI] [PubMed] [Google Scholar]
- 60. Lassovski M, Kyembe L, Maphalala G et al Decentralization of laboratory services for HIV patients` care in rural clinics in Shiselweni, Swaziland. In Cape Town, South Africa, 2013.
- 61. Jobanputra K, Parker LA, Azih C et al Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS ONE 2015: 10: e0116144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Keiser O, Chi BH, Gsponer T et al Outcomes of antiretroviral treatment in programmes with and without routine viral load monitoring in Southern Africa. AIDS Lond Engl 2011: 25: 1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gupta RK, Hill A, Sawyer AW et al Virological monitoring and resistance to first‐line highly active antiretroviral therapy in adults infected with HIV‐1 treated under WHO guidelines: a systematic review and meta‐analysis. Lancet Infect Dis 2009: 9: 409–417. [DOI] [PubMed] [Google Scholar]
- 64. World Health Organization . Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy, July 2017. World Health Organization: Switzerland, 2017. [PubMed] [Google Scholar]
- 65. Ford N, Migone C, Calmy A et al Benefits and risks of rapid initiation of antiretroviral therapy. AIDS 2018: 32: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dewing S, Mathews C, Fatti G, Grimwood A, Boulle A. Antiretroviral adherence interventions in Southern Africa: implications for using HIV treatments for prevention. Curr HIV/AIDS Rep 2014: 11: 63–71. [DOI] [PubMed] [Google Scholar]
- 67. Grimsrud A, Bygrave H, Doherty M et al Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J Int AIDS Soc 2016: 19: 21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Eaton EF, Saag MS, Mugavero M. Engagement in human immunodeficiency virus care: linkage, retention, and antiretroviral therapy adherence. Infect Dis Clin North Am 2014: 28: 355–369. [DOI] [PubMed] [Google Scholar]
- 69. Johnson LF, Estill J, Keiser O et al Do increasing rates of loss to follow‐up in antiretroviral treatment programs imply deteriorating patient retention? Am J Epidemiol 2014: 180: 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chi BH, Yiannoutsos CT, Westfall AO et al Universal definition of loss to follow‐up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med 2011: 8: e1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grimsrud AT, Cornell M, Egger M, Boulle A, Myer L. Impact of definitions of loss to follow‐up (LTFU) in antiretroviral therapy program evaluation: variation in the definition can have an appreciable impact on estimated proportions of LTFU. J Clin Epidemiol 2013: 66: 1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Setting.
Appendix S2. Diagnostic after multiple imputation.
Appendix S3. Building the flexible parametric survival model.
Appendix S4. Results.


