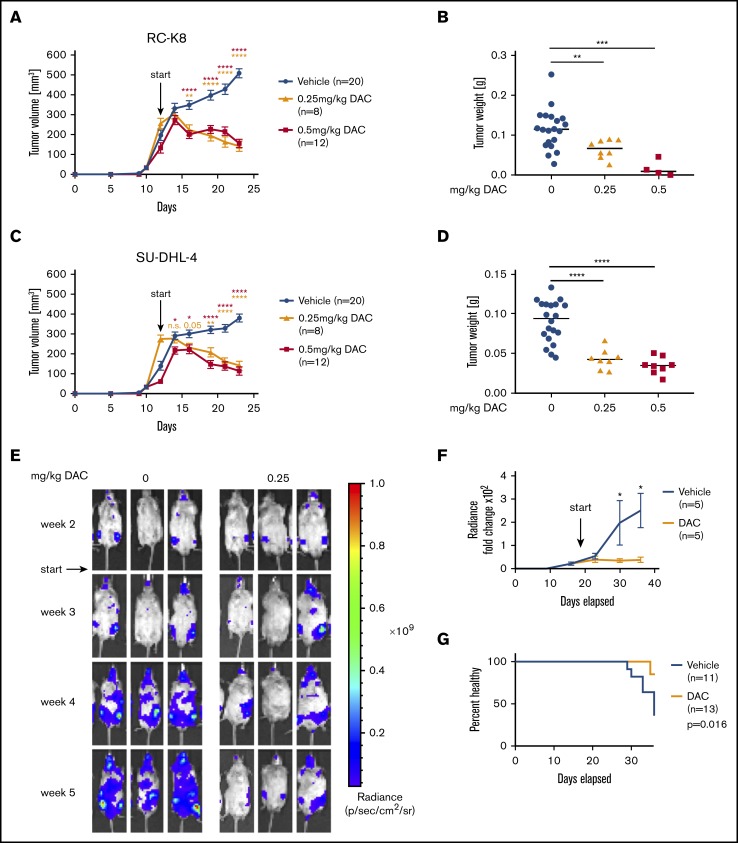
Figure 4.
DAC treatment decreases tumor burden in vivo in a subcutaneous and in a novel orthotopic model of DLBCL RC-K8 (A-B) and SU-DHL-4 (C-D) DLBCL cell lines were subcutaneously injected into the flanks of NSG mice. From day 12 after transplantation, mice were treated systemically with 0.25 mg/kg or 0.5 mg/kg DAC for 2 cycles, 5 days per week. Tumor volume was measured continuously (A,C), and tumor weight was measured at the end point (B,D). (B,D) Because of the toxicity of the 0.5-mg/kg treatment, only 4 of 12 mice and 8 of 12 mice are represented for RC-K8 and SU-DHL-4, respectively, at the end point. Graphs in panels A and C show mean ± SEM; in panels B and D, median values are presented as horizontal lines. Graphs represent pooled data from 2 independent experiments. The P values were calculated using the Mann-Whitney U test. (E-G) RC-K8 cells were subjected to lentiviral gene transfer of luciferase and injected IV into MISTRG6 mice. Mice were treated with 0 or 0.25 mg/kg DAC starting from day 19 after injection. Representative IVIS images of mice (E) and the radiance over 5 weeks (F) are shown. Results in panel F are from 1 experiment and representative of 2 independent ones. (G) Survival curve showing the percentage of injected mice without symptoms of disease (paralysis, weight loss); the P value was calculated using the log-rank (Mantel-Cox) test. Data are pooled from 2 independent experiments. *P < .05, **P < .01, ***P < .001, ****P < .0001. n.s., not significant.