Figure 3.
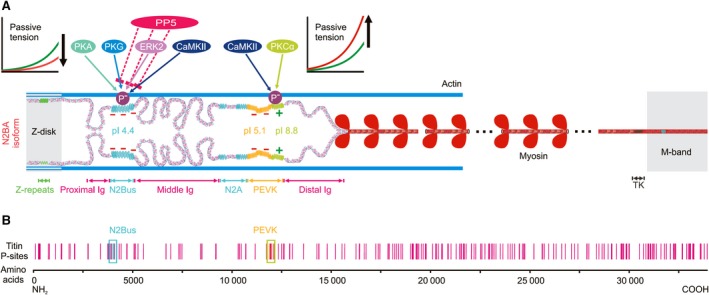
Potential and verified phosphorylation sites in human titin. (A) Layout of the N2BA titin isoform in a cardiac half‐sarcomere, highlighting protein kinases (for details, see main text) and protein phosphatase (PP)5 known to mediate phosphorylation/ dephosphorylation at two distinct molecular spring elements, N2Bus and constitutively expressed PEVK (light green bit of PEVK). Phosphorylation of N2Bus reduces titin‐based passive tension, whereas phosphorylation of PEVK increases it, which is explained by the different net charge of these elements. Constitutive PEVK has a net positive charge (+) and high isoelectric point (pI), N2Bus a net negative charge (−) and low pI. Note that the alternatively spliced PEVK subsegment (yellow bit of PEVK) also has a net negative charge. (B) Known potential phosphosites in human cardiac titin (vertical red bars), from http://www.phosphosite.org 85. Locations of phosphosites verified by site‐specific methods are highlighted (blue and green boxes). TK, titin kinase domain.
