Figure 1.
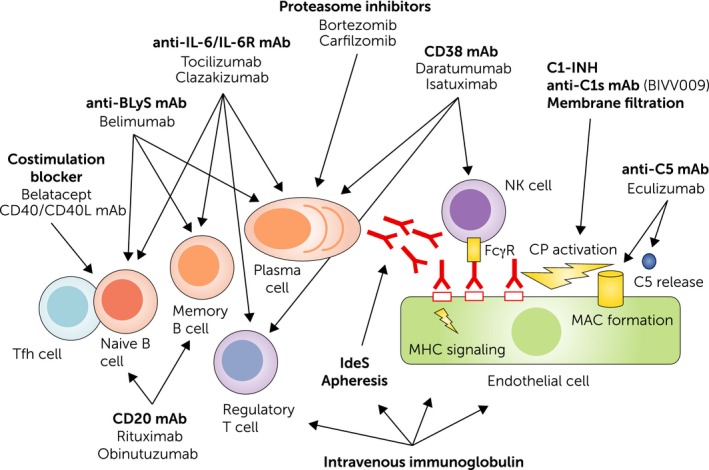
Pathogenesis of antibody‐mediated rejection and potential therapeutic targets. A primary trigger of B cell alloimmunity may be the interaction of follicular T helper cells with naive B cells. This leads to B cell proliferation and differentiation, and the generation of B memory cells and antibody‐producing plasma cells. Binding of alloantibodies to the endothelium may trigger direct signalling, induce Fc gamma receptor (FcγR) dependent cellular effects, such as natural killer (NK) cell (and macrophage) activation, and trigger complement activation via the classical pathway (CP). Costimulation blockers, monoclonal antibodies (mAb) that target the interleukin‐6 (IL‐6)/IL‐6 receptor (IL‐6R) axis, or B lymphocyte stimulator may prevent proper B cell activation/differentiation and affect the generation or integrity of plasma cells. IL‐6 antagonists may also enhance the formation of regulatory T cells. Proteasome inhibitors and CD38 mAb may deplete alloantibody‐producing plasma cells, the latter affecting also NK cells and regulatory T cells. Complement inhibitors and membrane filtration target the C1 complex, a key component of the CP, or by interference with the terminal component C5 (eculizumab), the formation of the membrane attack complex and anaphylatoxin C5a. The mode of action of intravenous immunoglobulin is multifaceted and may include interference with B and T cell activation, antibody formation and recycling, as well as complement activation.
