Abstract
Aim
To describe trends in walking and living independently in a cohort of consecutive cases of spina bifida, followed‐up over 50 years.
Method
From 1972 to 2017, a cohort of 117 (born 1963–1971, 50 males, 67 females) survivors and/or carers was surveyed approximately every 5 years by clinical examination and/or postal questionnaire/telephone interview. The Office for National Statistics provided details of deaths.
Results
The follow‐up in 2016 and 2017 was 99% (116/117). There were 37 survivors (17 males, 20 females) aged 46 to 53 years and 79 deaths (50y survival, 32%). The percentage of survivors who could walk more than 50m at the mean ages of 9 years, 18 years, 25 years, 30 years, 35 years, 40 years, 45 years, and 50 years was 51% (38/75), 50% (34/68), 33% (20/61), 30% (17/57), 30% (16/54), 30% (14/46), 31% (12/39), and 27% (10/37) respectively. However, the percentage living independently in the community after age 25 years increased over time: 23% (14/61); 37% (21/57); 41% (22/54); 39% (18/46); 56% (22/39); and 54% (20/37). Living independently at age 50 years was more common in survivors without a history of raised intracranial pressure or cerebrospinal fluid shunt revisions.
Interpretation
In this unselected cohort, mobility declined with age, possibly because of increasing obesity and deteriorating health. By contrast, partly because survival was better in those least disabled, the percentage living independently increased.
What this paper adds
By age 50 years, the percentage of patients who could walk more than 50m had declined to 27%.
By age 50 years, the percentage living independently had doubled to over 50%.
Survivors without a history of raised intracranial pressure or cerebrospinal fluid shunt revision are more likely to live independently.
What this paper adds
By age 50 years, the percentage of patients who could walk more than 50m had declined to 27%.
By age 50 years, the percentage living independently had doubled to over 50%.
Survivors without a history of raised intracranial pressure or cerebrospinal fluid shunt revision are more likely to live independently.
This article's abstract has been translated into Spanish and Portuguese.
Follow the links from the http://onlinelibrary.wiley.com/doi/10.1111/dmcn.14168/abstract to view the translations.
This article is commented on by Woodhouse on page https://doi.org/10.1111/dmcn.14170 of this issue.
Editor's Choice
My Editor’s Choice for the October 2019 issue reports the 50‐year follow‐up of the consecutive cohort of individuals with open spina bifida who underwent surgery at birth. It provides an important long‐term perspective through marked evolution in treatment, service, and attitudes towards disability, and emphasizes the need for a proactive lifelong outlook in developmental disability.
Video Podcast: https://www.youtube.com/watch?v=zJ5L5Zq66Yw
Resumen
Caminar y vivir de manera independiente cuando se tiene un diagnóstico de espina bífida: un estudio prospectivo de cohorte de 50 años
Objetivo
Describir las tendencias para caminar y vivir de forma independiente en una cohorte de casos consecutivos de espina bífida, seguidos durante 50 años.
Método
Desde 1.972 hasta 2.017, una cohorte de 117 (nacidos entre 1.963–1.971, 50 varones, 67 mujeres) sobrevivientes y/o cuidadores fueron encuestados aproximadamente cada 5 años mediante examen clínico y/o cuestionario postal/entrevista telefónica. La Oficina de Estadísticas Nacionales proporcionó detalles de las muertes.
Resultados
El seguimiento en 2.016 y 2.017 fue del 99% (116/117). Hubo 37 sobrevivientes (17 varones, 20 mujeres) de 46 a 53 años y 79 muertes (50 años de supervivencia, 32%). El porcentaje de sobrevivientes que pudieron caminar más de 50 metros en las edades medias de 9, 18, 25, 30, 35, 40, 45 y 50 años fue del 51% (38/75), 50% (34/68), 33% (20/61), 30% (17/57), 30% (16/54), 30% (14/46), 31% (12/39) y 27% (10/37) respectivamente. Sin embargo, el porcentaje de vida independiente en la comunidad después de los 25 años aumentó con el tiempo: 23% (14/61); 37% (21/57); 41% (22/54); 39% (18/46); 56% (22/39); y 54% (20/37). Vivir de forma independiente a los 50 años de edad fue más común en los sobrevivientes sin antecedentes de aumento de la presión intracraneal o revisiones de derivación del líquido cefalorraquídeo.
Interpretación
En esta cohorte no seleccionada, la movilidad disminuyó con la edad, posiblemente debido al aumento de la obesidad y al deterioro de la salud. Por el contrario, en parte porque la supervivencia fue mejor en los individuos con menos desafíos fisicos, el porcentaje de vida independiente aumentó.
Resumo
Caminhando e vivendo com independência tendo espinha bífida: um estudo de coorte prospectivo de 50 anos
Objetivo
Descrever tendências no caminhar e viver com independência em uma coorte de casos consecutivos de espinha bífida, acompanhados por 50 anos.
Método
De 1972 a 2017, uma coorte de 117 (nascidos 1963–1971, 50 do sexo masculino, 67 do sexo feminino) sobreviventes e/ou cuidadores foi avaliada aproximadamente a cada 5 anos por exame clínico e/ou entrevista por telefone ou correios. O Escritório de Estatística Nacional forneceu detalhes sobre óbitos.
Resultados
O acompanhamento em 2016 e 2017 foi 99% (116/117). Houve 37 sobreviventes (17 do sexo masculino, 20 do sexo feminino) com idades de 46 to 53 anos e 79 óbitos (sobrevivência em 50a, 32%). A porcentagem de sobreviventes que podiam andar mais de 50m nas idades médias de 9, 18, 25, 30, 35, 40, 45, e 50 foi 51% (38/75), 50% (34/68), 33% (20/61), 30% (17/57), 30% (16/54), 30% (14/46), 31% (12/39), and 27% (10/37) respectivamente. No entanto, a porcentagem vivendo independentemente na comunidade após a idade de 25 anos aumentou com o tempo: 23% (14/61); 37% (21/57); 41% (22/54); 39% (18/46); 56% (22/39); e 54% (20/37). Viver com independência na idade de 50 anos foi mais comum em sobreviventes sem história de aumento de pressão intra‐craniana ou revisões da válvula de líquido cérebro‐espinhal.
Interpretação
Nesta coorte não selecionada, a mobilidade diminuiu com a idade, possivelmente por causa do aumento da obesidade e deterioração das condições de saúde. Em contraste, em parte porque a sobrevivência foi melhor naqueles com menos incapacidades, a porcentagem dos que viviam com independência aumentou.
Abbreviation
- CSF
Cerebrospinal fluid
Myelomeningocele (a type of spina bifida) is the most common congenital abnormality causing physical disability.1 Almost 6000 affected infants were born in Europe between 1991 and 2011. Worldwide incidence is 0.2 to 6.4 per 1000 live births with higher rates in less developed countries.2, 3 Severity varies from no apparent disability to major health problems including paralysis, cognitive impairment, and bladder and bowel dysfunction.4, 5 Average lifetime cost of care is estimated at over €500 000.
Recently, there have been calls for reliable longitudinal data on trends in walking and living independently in patients with spina bifida.1, 4, 6 Doctors, parents, and health care planners want to know if children with a disability, such as spina bifida, will be able to look after themselves in adulthood.7 For example, will they achieve emerging adulthood milestones such as leaving home or gaining employment?8 Cross‐sectional studies fail to answer such crucial questions adequately, while most longitudinal studies have been of relatively short duration and/or had high loss to follow‐up.4, 9, 10 This is the rationale for the current study in which we followed up a cohort of patients with spina bifida from Cambridge, UK for 50 years. We describe trends in walking at the mean ages of 9 years 18 years 25 years, 30 years, 35 years, 40 years, 45 years, and 50 years. Since many young people live at home in early adulthood, we investigated trends in living independently from age 25 years onwards. We also explored possible predictors of walking or living independently at mean age 50 years.9, 10, 11, 12, 13, 14
Method
Participants
As previously described,11, 15, 16, 17 the cohort comprised 117 consecutive infants (50 males 67 females) with open myelomeningocele/spina bifida who were treated unselectively at Addenbrooke's Hospital, Cambridge, UK from 1963 to 1971. After a meticulous neurological examination, all had their backs closed within 48 hours of birth;15 if required, a cerebrospinal fluid (CSF) shunt was inserted. This was done in 89% (82/92) of infants who reached the age of 1 year. Until 2012, the cohort had been reviewed about every 5 years with no loss to follow‐up.11, 16, 17, 18, 19 The reviews at home and school at the mean ages of 4 years and 9 years included clinical examination; in later reviews, only those still attending Addenbrooke's Hospital (51% 35/69 at mean age 18y) were examined. Later reviews were based mainly on questionnaire surveys and clinical records. The National Research Ethics Service Committee East of England‐Cambridge East reviewed the study (reference 07//Q0104/11).
Data collection
In 2016 and 2017, we conducted a confidential postal questionnaire survey of survivors backed by a telephone interview with the patient and/or carer. Carers included parents, partners, and/or health care staff. To ensure comparability, we used the same questions as in previous reviews.16, 17, 19 Details of deaths up to August 2017 were obtained from the Office for National Statistics and the Health and Social Care Information Centre (flagged cohort MR564), backed by information from medical records and autopsy reports.17
Statistical analysis
We defined walking as being able to walk more than 50m independently outside with walking aids such as walking sticks, crutches, callipers, or rollators, if needed.18 We defined living independently as living without supervision in the community, coping independently with housekeeping, meals, all personal care needs, and transport. This could include hiring a cleaner but did not include independence in a sheltered environment where support can be provided if needed.10, 11
Using data from the 2016 and 2017 review and previous reviews,11, 15, 16, 17, 18, 19, 20 we analysed trends in walking at the mean ages of 9 years 18 years, 25 years, 30 years, 35 years, 40 years, 45 years, and 50 years, and trends in living independently from mean age 25 to 50 years. Previous studies suggested that walking and survival were related to the level of the lesion,5, 12, 14, 21 whereas living independently was related to CSF shunt history.11 We used Fisher's exact test to explore possible predictors of walking and living independently at age 50 years. Survival may be better in those less disabled who have a lower sensory level.12, 16 We used the logrank test with Kaplan–Meier curves to compare survival in those born with a sensory level below L3 versus the remainder.
Results
Follow‐up in 2016 and 2017 was 99% (116/117). One individual known to be alive in the Middle East in 2013 could no longer be contacted. There were 37 survivors of whom 36 were interviewed by telephone (three supported by a parent or health care worker), and one questionnaire was completed by a parent. There had been 79 deaths. Causes of death were: cardiorespiratory (26); neurological (24: hydrocephalus [10]; central nervous system infection [10]; epilepsy [4]); urological (22); and other causes (7: inhaled vomit [2]; sudden infant death syndrome [1]; thrombocytopenic purpura [1]; carcinoma of the cervix [1]; malignant melanoma [1]; sepsis [1]). Forty members of the cohort (34%) had died before the age of 5 years (25 before their first birthday) and 39 (33%) during the following 45 years. The 50‐year survival rate was 32% (37/116).
The mean age of the 37 survivors was 50 years (median: 50y; range: 46–53y) 17 were males and 20 were females. Thirty‐one survivors had a CSF shunt. In 11, the shunt had never been revised, eight had revisions before the age of 2 years, and 12 after 2 years (median number of revisions in these 20 survivors: 2; range: 1–4). Fourteen survivors had a history of clinically documented symptoms or signs of raised intracranial pressure.
Most survivors (29/37) had an IQ of more than 80 in childhood (range: 51–137), and seven were fully continent (without aids). Around half (20/37) had a sensory level at birth below L3, 18 had a motor level below L3, and 26 had quadriceps activity recorded in infancy. Five worked in open/competitive paid employment (three using wheelchairs) and two worked in sheltered employment. Thirteen reported chronic pain in the head, neck, or back.
Walking
Figure 1 shows that the percentage of survivors who could walk at least 50m at the mean ages of 9 years, 18 years, 25 years, 30 years, 35 years, 40 years, 45 years, and 50 years was 51% (38/75), 50% (34/68), 33% (20/61), 30% (17/57), 30% (16/54), 30% (14/46), 31% (12/39), and 27% (10/37) respectively. Of 38 who were defined as walkers at the mean age of 9 years, 20 (53%) lost the ability to walk 50m as they became older. Table 1 shows that the ability to walk at age 50 years could be predicted from assessing sensory level, motor level, or quadriceps activity at birth. However, walking was not significantly related to IQ or CSF shunt history.
Figure 1.
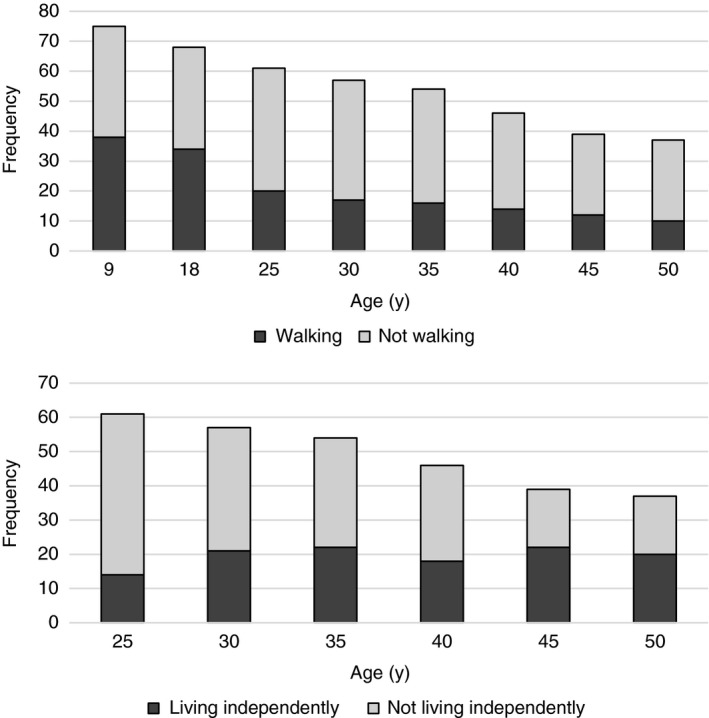
Number of survivors walking and/or living independently according to age. One hundred per cent follow‐up except at mean age 50y when we could not contact one minimally affected case (with an S1 sensory level) who had been walking and living independently in 2013. Walking is defined as being able to walk at least 50m independently outside with walking aids if needed,11 except at mean age 9y when walking is defined as ‘walks independently’ or ‘sometimes uses wheelchair’. Living independently is defined as living without supervision in the community, and coping independently with housekeeping, all personal needs, and transport.
Table 1.
Possible predictors of being able to walk more than 50m at age 50y in 37 survivors with open spina bifida
| Predictor | Proportion with predictor who could walk (%) | Proportion without predictor who could walk (%) | p |
|---|---|---|---|
| Birth sensory level below L3 | 9/20 (45) | 1/17 (6) | 0.017 |
| Birth motor level below L3 | 8/18 (44) | 2/19 (11) | 0.048 |
| Birth quadriceps activity | 10/26 (38) | 0/11 (0) | 0.031 |
| No CSF shunt or shunt revision | 7/17 (41) | 3/20 (15) | 0.157 |
| IQ at school ≥80 | 9/29 (31) | 1/8 (13) | 0.575 |
CSF, cerebrospinal fluid.
Living independently
The percentage of survivors who lived independently in the community at the mean ages of 25 years 30 years 35 years, 40 years, 45 years, and 50 years was 23% (14/61), 37% (21/57), 41% (22/54), 39% (18/46), 56% (22/39), and 54% (20/37) respectively. Over half (55%, 11/20) of those living independently at age 50 years used wheelchairs.
Most of the cohort (67%, 41/61) showed no change in independence over their lifespan (10 living independently, 31 not living independently). However, 12 improved (seven at age 30y, two at age 40y, three at age 45y), two got worse, and six fluctuated over time. Altogether, nearly half (49%, 30/61) of those who survived to age 25 years and beyond lived independently at some point. Table 2 shows that compared to those needing daily care, survivors who lived independently at the mean age of 50 years were less likely to have had clinically documented symptoms of raised intracranial pressure or CSF shunt revisions, and were more likely to be able to walk.
Table 2.
Possible predictors of living independently at age 50y in 37 survivors with open spina bifida
| Predictor | Proportion with predictor who live independently (%) | Proportion without predictor who live independently (%) | p |
|---|---|---|---|
| No history of raised intracranial pressure | 17/23 (74) | 3/14 (21) | 0.005 |
| No CSF shunt or shunt revision | 14/17 (82) | 6/20 (30) | 0.004 |
| IQ at school ≥80 | 18/29 (62) | 2/8 (25) | 0.143 |
| Can walk ≥50m at age 50y | 9/10 (90) | 11/27 (41) | 0.017 |
| Birth sensory level below L3 | 13/20 (65) | 7/17 (41) | 0.264 |
| Birth motor level below L3 | 11/18 (61) | 9/19 (47) | 0.612 |
CSF, cerebrospinal fluid.
Overall, median survival for the whole cohort was 29 years. Figure 2 shows that survival at the mean age 50 years was better in those born with sensation to pinprick below the knee (sensory level below L3) compared to those born with a more extensive neurological deficit: 54% (20/37) in those with a sensory level below L3 versus 22% (17/79) in those with a sensory level of L3 and above (logrank p=0.001).
Figure 2.
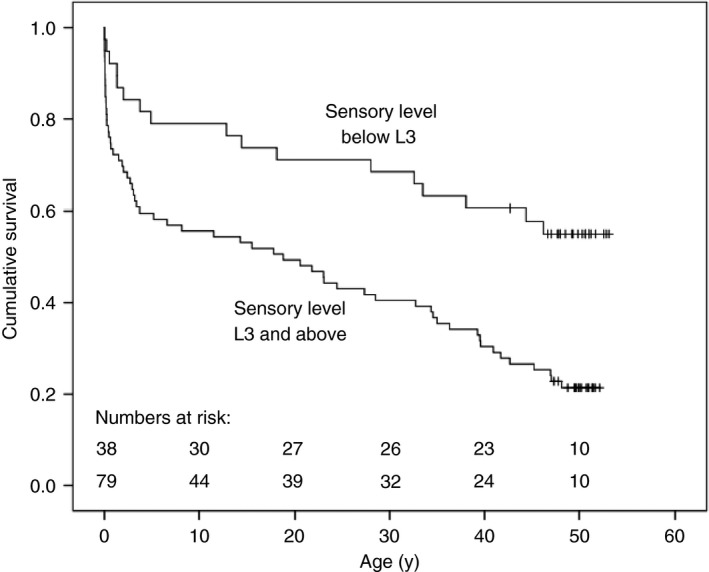
Fifty‐year survival and neurological deficit at birth in 117 consecutive, unselected cases of open spina bifida. Those born with a sensory level below L3 were more likely to survive than those with a higher sensory level (54% vs 22%, logrank p=0.001) at mean age 50y. One minimally affected case (with an S1 sensory level) was lost to follow‐up after the age of 43y. At age 50y, results have been censored for 17 cases who were alive (aged 46–49y) in August 2017.
Discussion
Principal findings
The percentage of survivors with spina bifida who could walk more than 50m declined from around half in childhood and adolescence to a quarter at age 50 years. However, partly because of the increased mortality in those severely affected, the percentage living independently in the community increased from around 25% at age 25 years to over 50% at age 50 years. Living independently was more likely in those who had never had symptoms of raised intracranial pressure.
Strengths and limitations
This is the first cohort study to describe trends in walking and living independently in patients with spina bifida up to the mean age of 50 years. The long duration and over 99% follow‐up make this study unique.21 The approach and findings are novel (and different from previous reviews of the same cohort) because we used longitudinal data on mobility and independence from eight consecutive assessments from 1985 to 2017. The study has other strengths. This was an unselected cohort with a wide range of disabilities. Neurological examination in infancy was consistent and findings are at a relatively low risk of bias because follow‐up was done independently by clinicians not involved in patient management.
The main limitation is that improvements in medical management mean that our findings may not reflect the prognosis for individuals born more recently. However, our results are surprisingly similar to the postnatal repair group in a recent 2011 trial, which compared prenatal and postnatal repair of myelomeningocele:4 CSF shunt insertion by age 12 months was 82% compared to 89% in our cohort; independent walking was recorded in 57% (38/67) of patients at age 2 years 6 months versus 51% (40/79) at age 9 years in our cohort.15 Additionally, walking rates in late adolescence were comparable to the only other unselected prospective cohort study of spina bifida:13, 21 46% (33/71) at age 21 years versus 50% at age 18 years in our cohort.
Other weaknesses include: the small size of the cohort, although this is comparable with other studies.4, 13 Later reviews were mainly by telephone/postal questionnaire rather than objective clinical assessment because many survivors had moved away from Cambridge. Findings may not be generalizable to different systems of medical care or to those of non‐white ethnicity. Finally, this is a largely descriptive analysis. The associations explored were based on clinically likely effects,11, 12, 13 but we cannot rule out potential confounders for which we were unable to adjust due to the small sample size.
Comparison with other studies
As described earlier, our results in childhood/adolescence appear similar to more recent US studies of spina bifida that started at or before birth, were non‐selective, and finished recruiting in 197913 and 20104 respectively.
Our results for mobility are comparable to a large cross‐sectional study of patients enrolled in a US national spina bifida patient registry, which found 45% were community walkers at the mean age of 15 years,1 and to a smaller study where 31% walked at the mean age of 30 years.9
Previous reports on living independently in adults with spina bifida6, 9, 10 include cross‐sectional studies of convenience samples with mean ages of 21 to 31 years and response rates of only 34% to 50%. These studies found that 24% (12/50) were living independently at the mean age of 21 years,6 and 30% (25/84) at the mean age of 31 years,9 which is comparable to our study. The authors of the studies highlighted the need to investigate independent living using community‐based studies rather than mainly focusing on those attending hospital clinics.
Implications
Our findings could influence clinical practice. The percentage of adults with spina bifida who could walk more than 50m declined over time, possibly because of deteriorating health and increasing obesity.13, 14 In our cohort, the proportion who reported they were overweight or obese more than doubled from 21% (13/61) at age 25 years to 44% (24/54) at age 35 years. Weight management and/or exercise programmes might improve mobility outcomes.1, 5, 22
Second, since a history of raised intracranial pressure and/or CSF shunt revisions was associated with reduced independence, doctors should consider prompt neurosurgical referral of patients with symptoms.23 Patients and carers need to be aware of possible red flag symptoms including headaches, nausea, drowsiness, unsteadiness, and/or visual problems. Shunt malfunction, often unrecognised, is a potentially avoidable cause of death and disability.5, 21 Prompt neurosurgical assessment and treatment might preserve function and independence.13
One reason for the increase over time in the percentage of patients living independently is because survival was better in those who were least disabled17, 24 and had a lower sensory level at birth. However, the 14% increase in those living independently between the ages of 25 years and 30 years might be partly related to the 1990s UK government policy promoting ‘care in the community’. This involved encouraging people with disabilities to live at home in specially adapted accommodation with visiting support as needed; it also resulted in the closure of large residential institutions. In addition, some of those dependent on their parents as young adults later began living independently when their parents died or became incapacitated. Increasing use of the Internet and mobile phones may also have made it easier for some individuals to live independently.
Whether or not they live independently, patients with spina bifida are also at high risk of sudden, unexpected death.25 Nearly half (46% 18/39) of those in our cohort who died after the age of 5 years died suddenly, and almost all these deaths were followed by a coroner's postmortem. The most common causes were epilepsy, acute hydrocephalus, pulmonary embolus, or acute renal sepsis.17 Sleep apnoea and mid‐brain elongation may also be risk factors for sudden death.25
This study may help families and health professionals to have realistic expectations of long‐term outcomes in spina bifida. The two major factors predicting disability are the neurological deficit at birth and a history of CSF shunt.2, 10, 11, 12, 14 A less extensive neurological deficit/low sensory level is associated with better long‐term survival, mobility, continence, and cognitive ability, and a reduced risk of death caused by urological disorders.14, 15, 16, 19 Not needing a CSF shunt, or not needing a shunt revision, is associated with independence.5, 11, 21 Finally, the unexpected finding of a doubling over time in the percentage of survivors living independently might be widely applicable (e.g. cerebral palsy) and deserves further research.
Acknowledgements
We thank the patients and their carers. We also thank Cathy Mackay and Sarah Kerry‐Barnard. The Health and Social Care Information Centre/Office for National Statistics provided information on deaths. The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
References
- 1. Dicianno BE, Karmarkar A, Houtrow A, et al. Factors associated with mobility outcomes in a national spina bifida patient registry. Am J Phys Med Rehabil 2015; 94: 1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bowman RM, Boshnjaku V, McLone DG. The changing incidence of myelomeningocele and its impact on pediatric neurosurgery: a review from the Children's Memorial Hospital. Childs Nerv Syst 2009; 25: 801–6. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell LE, Adzick NS, Melchionne J, Pasquariello PS, Sutton L, Whitehead AS. Spina bifida. Lancet 2004; 364: 1885–95. [DOI] [PubMed] [Google Scholar]
- 4. Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011; 364: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thompson DN. Postnatal management and outcome for neural tube defects including spina bifida and encephalocoeles. Prenat Diagn 2009; 29: 412–9. [DOI] [PubMed] [Google Scholar]
- 6. Bellin MH, Dicianno BE, Levey E, et al. Interrelationships of sex, level of lesion, and transition outcomes among young adults with myelomeningocele. Dev Med Child Neurol 2011; 53: 647–52. [DOI] [PubMed] [Google Scholar]
- 7. Holbein CE, Zebracki K, Bechtel CF, Lennon Papadakis J, Franks Bruno E, Holmbeck GN. Milestone achievement in emerging adulthood in spina bifida: a longitudinal investigation of parental expectations. Dev Med Child Neurol 2017; 59: 311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zukerman JM, Devine KA, Holmbeck GN. Adolescent predictors of emerging adulthood milestones in youth with spina bifida. J Pediatr Psychol 2011; 36: 265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roach JW, Short BF, Saltzman HM. Adult consequences of spina bifida: a cohort study. Clin Orthop Relat Res 2011; 469: 1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verhoef M, Barf HA, Post MW, van Asbeck FW, Gooskens RH, Prevo AJ. Functional independence among young adults with spina bifida, in relation to hydrocephalus and level of lesion. Dev Med Child Neurol 2006; 48: 114–9. [DOI] [PubMed] [Google Scholar]
- 11. Hunt GM, Oakeshott P, Kerry S. Link between the CSF shunt and achievement in adults with spina bifida. J Neurol Neurosurg Psychiatry 1999; 67: 591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verhoef M, Barf HA, Post MW, van Asbeck FW, Gooskens RH, Prevo AJ. Secondary impairments in young adults with spina bifida. Dev Med Child Neurol 2004; 46: 420–7. [DOI] [PubMed] [Google Scholar]
- 13. Bowman RM, McLone DG, Grant JA, Tomita T, Ito JA. Spina bifida outcome: a 25‐year prospective. Pediatr Neurosurg 2001; 34: 114–20. [DOI] [PubMed] [Google Scholar]
- 14. Woodhouse CR. Myelomeningocele in young adults. BJU Int 2005; 95: 223–30. [DOI] [PubMed] [Google Scholar]
- 15. Hunt G, Lewin W, Gleave J, Gairdner D. Predictive factors in open myelomeningocele with special reference to sensory level. Br Med J 1973; 4: 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oakeshott P, Reid F, Poulton A, Markus H, Whitaker RH, Hunt GM. Neurological deficit at birth predicts survival to the mid‐40s and urological deaths in open spina bifida: a complete prospective cohort study. Dev Med Child Neurol 2015; 57: 634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oakeshott P, Hunt GM, Poulton A, Reid F. Expectation of life and unexpected death in open spina bifida: a 40‐year complete, non‐selective, longitudinal cohort study. Dev Med Child Neurol 2010; 52: 749–53. [DOI] [PubMed] [Google Scholar]
- 18. Hunt GM, Poulton A. Open spina bifida: a complete cohort reviewed 25 years after closure. Dev Med Child Neurol 1995; 37: 19–29. [DOI] [PubMed] [Google Scholar]
- 19. Hunt GM, Oakeshott P. Outcome in people with spina bifida at age 35: prospective community based cohort study. BMJ 2003; 326: 1365–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunt GM. Open spina bifida: outcome for a complete cohort treated unselectively and followed into adulthood. Dev Med Child Neurol 1990; 32: 108–18. [DOI] [PubMed] [Google Scholar]
- 21. Piatt JH Jr. Treatment of myelomeningocele: a review of outcomes and continuing neurosurgical considerations among adults. J Neurosurg Pediatr 2010; 6: 515–25. [DOI] [PubMed] [Google Scholar]
- 22. Şimşek TT, Türkücüoğlu B, Tezcan S. Examination of the relationship between body mass index (BMI) and functional independence level in children with spina bifida. Dev Neurorehabil 2015; 18: 149–54. [DOI] [PubMed] [Google Scholar]
- 23. Shastin D, Zaben M, Leach P. Life with a cerebrospinal fluid (CSF) shunt. BMJ 2016; 355: i5209. [DOI] [PubMed] [Google Scholar]
- 24. Oakeshott P, Hunt GM. Long‐term outcome in open spina bifida. Br J Gen Pract 2003; 53: 632–6. [PMC free article] [PubMed] [Google Scholar]
- 25. Jernigan SC, Berry JG, Graham DA, et al. Risk factors of sudden death in young adult patients with myelomeningocele. J Neurosurg Pediatr 2012; 9: 149–55. [DOI] [PubMed] [Google Scholar]


