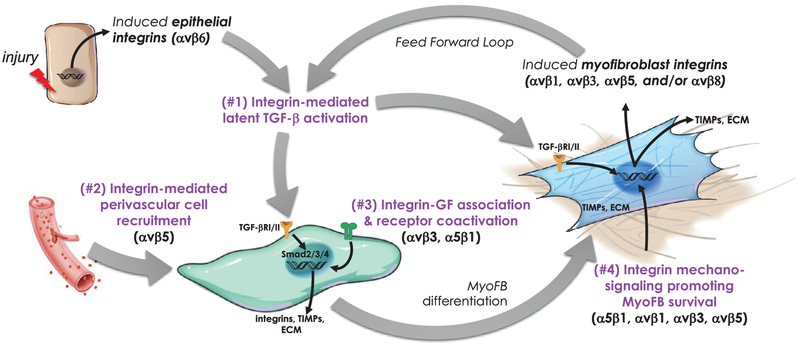
Fig 1.
Model illustrating 4 potential roles of RGD-binding integrins in the initiation, development, and perpetuation of organ fibrosis.1 Acute or chronic tissue injury locally upregulates the expression of integrins such as αvβ6 on epithelial cells which is a potent activator of the latent TGF-β stored in the surrounding ECM. The activated cytokine binds to its receptor on unactivated fibroblasts and initiates a SMAD-dependent signaling pathway culminating in the induction of dozens of target genes including the subunits of other RGD integrins, collagen, and other fibrotic matrix components, inhibitors of matrix degradation, and a constellation of other factors that promote transdifferentiation to a myofibroblastic phenotype.2 Evidence suggests myofibroblasts have a predominantly perivascular origin and that αvβ5 may have an important role in their recruitment to sites of developing fibrosis in kidney.3 Growth factors such as CTGF are capable of interacting with integrins on the cell surface and may provide cooperative signaling with TGF-β in the developing fibrotic response. Myofibroblasts exhibit expression of additional αv-containing integrins capable of activating more latent TGF-β setting up a feed forward loop.1,4 The myofibroblast integrins also act as sensors of ECM composition and stiffness which is important for maintaining their phenotype and survival, thus preserving the fibrotic niche and inhibiting resolution. ECM, extracellular matrix; myoFB, myofibroblast; TIMPs, tissue inhibitor of metalloproteases; TGF-βR1/RII, transforming growth factor receptor-I/II.