Abstract
Trace elements play an important role in tuberculosis infection because their deficiencies can be associated with impaired immunity. Blood samples were collected from a total of 320 active pulmonary tuberculosis patients and healthy individuals. The serum concentrations of Zinc, Iron, Copper, Calcium, lead, Arsenic and Selenium were analyzed by atomic absorption spectrometry. The levels of trace elements were measured after 2, 4 and 6 months of anti-TB treatment initiation in TB infected groups. Compared to the control group, the concentrations of Zinc, Selenium, and Iron were significantly lower (P < 0.001) in tuberculosis patients; however, that of Arsenic, Lead, and copper was significantly higher (P < 0.001) in the serum of patients. Cu/Zn and Cu/Se ratios were also significantly higher (P < 0.001) in TB patients compared to the control group. In addition, serum concentration calcium was similar in both TB patients and healthy controls. Our results indicated that trace elements concentrations in tuberculosis patients are related to each element role in immune system. Wherever the element is essential for the pathogenesis of bacteria, its concentration will remain low; and contrariwise, when the element is toxic for the bacteria, its level will be regulated up to provide a perfect condition for bacterial growth.
Keywords: Trace elements; Blood; Atomic absorption spectroscopy; Sistan, Pulmonary tuberculosis; Trace elements; Graphite furnace atomic absorption spectrometry; Sistan
1. Introduction
Tuberculosis (TB) is a disease caused by the bacterium Mycobacterium tuberculosis. TB spreads from person to person through tiny droplets released into the air when infected people who have active TB cough or sneeze. Mycobacterium tuberculosis infects about one-third of the world's population, of whom more than 80% live in developing countries like Iran. Only 57% of all patients with TB are diagnosed in Iran. [1], [2] Since the TB diagnostic tests are not highly sensitive to confirm the effectiveness of antitubercular therapy in some patients, especially in elderly patients, the symptoms might result in false positive results. [3]
Trace elements such as iron (Fe), copper (Cu), lead (Pb), arsenic (As), selenium (Se), calcium (Ca) and zinc (Zn) are essential nutrients and play an important role in many biological systems. [4], [5], [6], [7] The functions of these metals are crucial to the maintenance and growth of human tissues and organs. [8], [9] Moreover, these elements now have been determined as a potential key factor in many infectious diseases. A set of evidences indicated that some transition metals help immune system suppress microorganisms such as Mycobacterium tuberculosis. [10], [11]
Iron is necessary for M. tuberculosis growth in macrophages, and increased iron intake is associated with developing active tuberculosis infection and its mortality. [12], [13], [14] Several studies have suggested that the excess iron in human body assists Mycobacterium tuberculosis and HIV multiplication which can lead to a HIV-TB co-infection in many patients. [15], [16], [17], [18] The role of iron in the pathogencity, growth and metabolism of M. tuberculosis depends on the acquisition of iron from host resources, therefore high prevalence of iron deficiency anemia is usually observed among TB infected patients. [19], [20]
Zinc is used by the cells of the immune system to destroy bacteria such as tubercle bacilli or Escherichia coli. [21], [22] The lower levels of zinc in blood sample of TB and TB- HIV infected patients have been reported in some previous studies. [3], [23], [24] Many bacteria such as M. tuberculosis have DNA-binding metal-responsive transcriptional repressors that can regulate the transcription process. [25] Lead (Pb) is one of those metals able to alleviate the repression process so that it promotes the mycobacterium transcription. [26]
The role of copper in biological systems originates from its presence in the structure of many enzymes that act in oxidative and reductive reactions, such as cytochrome oxidase, lysyl oxidase, superoxide dismutase, ceruloplasmin, and metallothioneins. [27] Although copper has many biological benefits, its overload is toxic, and could inhibit M. tuberculosis growth. [28] In a study on pigs, TB infection was controlled by increasing copper concentration in lung lesions. [29]
It is believed that arsenic is an immunosuppressant and also a cause of chronic lung disease. Hence, it may intensify the TB infection. Smith et al. showed that the mortality from pulmonary tuberculosis was increased in arsenic-exposed individuals in the Chilean cohort. [30]
Selenium is an essential trace element which is important in immunity against microorganisms. However, blood selenium levels are lower in patients who suffer pulmonary tuberculosis and TB-HIV infection. [31] Higher mortality among TB infected patients is related to low levels of blood selenium. [32] Consumption of Selenium-Vitamin E supplements has been highly recommended due to its potential to decrease the reactive oxygen species and increase antioxidant activities in pulmonary tuberculosis patients. [33]
Hypocalcemia is a problem in tuberculosis which is related to poor nutritional status, therefore calcium phosphate agents are proposed to be used during anti-TB therapy. [34]
Heavy metals have some effects on the respiratory tract, epithelial cells, endothelial cells, and alveolar macrophages. [35] Immune system has different and complex interactions and there are many targets for metals to affect the functions of the cells which are involved in Immune response. [36], [37]
Given the lack of information regarding the levels of trace elements in pulmonary tuberculosis patients in Iran, we aimed to investigate the serum concentrations of Cu, Zn, Ca, Se, As, Pb and Fe in both TB patients and healthy controls.
2. Materials and methods
2.1. Study groups
This cross sectional study was carried out on 320 subjects, and two groups were formed: patient and healthy. Of the total of 320 samples, there were 160 normal healthy individuals and 160 patients with active TB. All the TB samples were collected from difinitly diagnosed pulmonary tuberculosis cases attending TB centers and hospitals in Sistan, Iran. Patients with another diagnosed pulmonary co-morbidity such as asthma, pneumonia, chronic obstructive pulmonary disease (COPD) and pulmonary malignancies were excluded. Healthy individuals were selected using probability sampling. Therefore, first Zabol (the sampling region) was divided into six areas. Then, we picked two zones in each area. We referred to the homes, and asked about exposure to TB or other pulmonary diseases. All of the subjects were physically examined by a trained physician. Finally, we selected 15 healthy subjects from each zone. We obtained written consent after complete explanation about the study from healthy individuals, tuberculosis patients or their parents or legal guardians. It was done in accordance with the Declaration of Helsinki. The local medical ethical committee has approved the study.
2.2. Sample preparation
Blood was collected into vacutainer tubes without any additives. Immediately after collection, each blood tube was centrifuged at 2500 rpm for 15 min in order to separate blood cells and serum. The sera were poured into polyethylene tubes and kept frozen at −20 °C until the time for the element analysis.
2.3. Determination of trace elements in serum
In order to determine the levels of Cu, Zn, Ca, Se, As, Pb and Fe in the serum samples, 10 ml of concentrated nitric acid was added to 1 ml of serum in a beaker and heated below for 3 hours, below boiling point, on a hot plate. When the volumes of the samples reduced to about one-third, 5 ml of 30% hydrogen peroxide solution was added. The samples were further heated almost to dryness at the same temperature. Finally, the residues were dissolved in 50 ml of 1% nitric acid and were filtered.
The prepared samples were transferred into 50 ml polyethylene tubes for trace element analysis. To measure Cu, Zn, Ca, Pb and Fe Flame Atomic Absorption Spectrometry (FAAS) (model WFX-210, Rayleigh, China), and to measure Se and As Hydride Generation Atomic Absorption Spectroscopy (HGAAS) (model WFX-210, Rayleigh, China) were used. [38], [39]
2.4. Statistical analysis
Statistical analysis was carried out using SPSS version 18 statistical package for social sciences (SPSS Inc., Chicago, IL, USA). [40], [41], [42] The main instrument parameters are shown in Table 1. Mean concentrations of the elements were compared between cases and controls using independent T test (for normally distributed variables) or Mann Whitney U test (for non-normally distributed variables). P-value less than 0.05 was considered statistically significant.
Table 1.
The main instrument parameters.
| Flame atomic absorption spectrometry (FAAS) | ||||||
|---|---|---|---|---|---|---|
| Element | Wavelength(nm) | Slit width (nm) | Lamp current (mA) | Burner height (mm) | Acetylene flow (L/min) | Air flow (L/min) |
| Cu | 324.8 | 0.5 | 4 | 10 | 1.70 | 13.5 |
| Zn | 213.9 | 1.0 | 4 | 10 | 2.00 | 13.5 |
| Fe | 248.3 | 0.2 | 8 | 10 | 2.50 | 13.5 |
| Pb | 283.3 | 0.7 | 4 | 10 | 2.5 | 13.5 |
| Hydride generation atomic absorption spectroscopy (HGAAS) | ||||||
| NaBH4 concentration | 0.5% | |||||
| NaOH concentration | 1M | |||||
| NaOH/NaBH4 flow-rate | 0.33 ml min-1 | |||||
| HCl concentration | 5% | |||||
| HCl flow-rate | 0.33 ml min-1 | |||||
| Sample flow-rate | 0.33 ml min-1 | |||||
| Wavelength(nm) for As | 193.7 nm | |||||
| Wavelength(nm) for Se | 196.0 nm | |||||
3. Results
A total of 160 tuberculosis patients aged between 17 and 87 years were included in this study. Out of all TB patients, 95 cases (52.7%) were males and 85 cases (47.3%) were females. Mean±SD BMI was 21.3 Kg/m2.Mean serum levels of Cu, Zn, Ca, Se, As, Pb and Fe were 102 ± 9.8 (µg/dl), 72 ± 9.6 (µg/dl), 12 ± 1.2 (mg/dl), 55 ± 16.8 (µg/dl), 5.5 ± 2.8 (µg/dl), 26 ± 6.5 (µg/dl) and 54 ± 12.9 (µg/dl) respectively.
Totally 160 normal healthy volunteers aged between 12–85 years were entered to our experiment. Of 160 healthy controls, 87 cases (48%) were males and 93 cases (52%) were females. Mean of BMI for this group was 21.04 Kg/m2. As shown in Table 2, the mean ± SD. Concentrations of Cu, Zn, Ca, Se, As, Pb and Fe were found to be 91 ± 7.5 (µg/dl), 95 ± 8.4 (µg/dl), 13 ± 0.9 (mg/dl), 76 ± 13.2 (µg/dl), 3.52 ± 1.0 (µg/dl), 10.8 ± 4.3 (µg/dl) and 78 ± 17.6 (µg/dl) respectively.
Table 2.
The means of the elements in the blood of the patients and control groups.
| Trace elements | Control group (mean ± SD) | Patients whit TB (mean ± SD) |
|---|---|---|
| N = 160 | N = 160 | |
| Cu (µg/dl) | 91 ± 7.5 | 102 ± 9.8 |
| Zn (µg/dl) | 95 ± 8.4 | 72 ± 9.6 |
| Ca (mg/dl) | 13 ± 0.9 | 12 ± 1.2 |
| Se (µg/dl) | 76 ± 13.2 | 55 ± 16.8 |
| As (µg/dl) | 3.52 ± 1.0 | 5.5 ± 2.8 |
| Pb (µg/dl) | 10.8 ± 4.3 | 26 ± 6.5 |
| Fe (µg/dl) | 78 ± 17.6 | 54 ± 12.9 |
| Cu/Zn | 0.9 ± 0.8 | 1.44 ± 0.2 |
| Cu/Se | 0.8 ± 0.6 | 2.04 ± 0.7 |
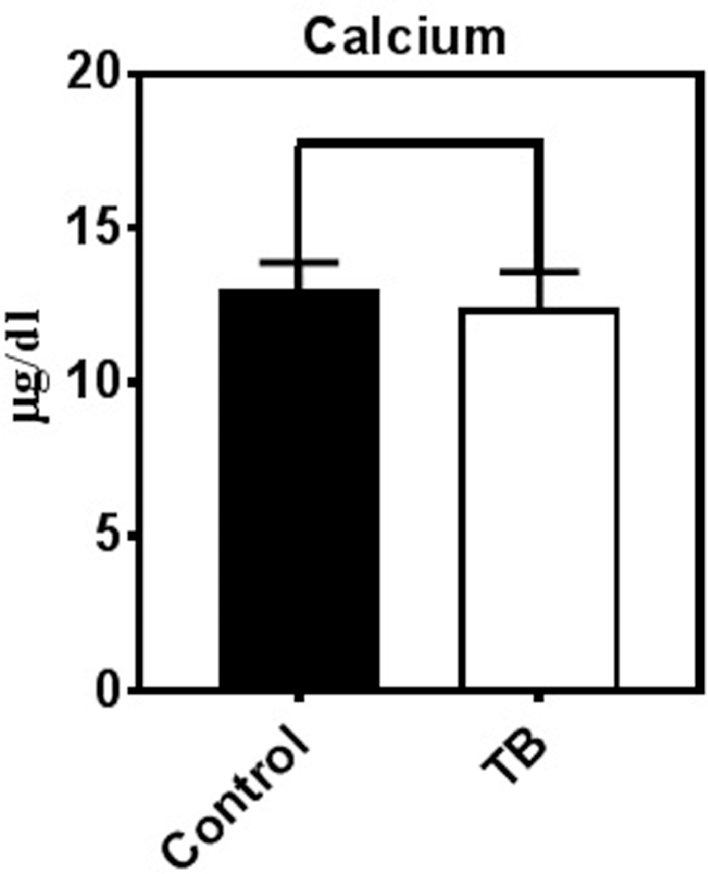
We observed that the mean values of trace elements in sera of TB patients and healthy controls were different. The mean ± S.D. concentrations of zinc, Selenium, and Iron were significantly lower in TB patients comparing with the control group (P < 0.001) (Fig. 1). On the other hand, the mean ± SD serum concentrations of arsenic, lead and copper were significantly higher in the patients compared with the control group (P < 0.001) (Fig. 2). Moreover, Cu/Zn and Cu/Se ratios were significantly higher in TB patients compared to the control group (P < 0.001) (Fig. 3). We also found that there was no significant difference between serum calcium in TB infected patients and control groups (P > 0.05) (Fig. 4)
Fig. 1.
Biometals which were decreased in TB patients. Zinc, Selenium, and Iron ion was measured in control (n = 160) and TB patients (n = 160) groups using Flame Atomic Absorption Spectrometry. The results 0showed that Zinc (A), Selenium (B), and Iron (C) ions are significantly decreased in TB patients (P < 0.001, ***).
Fig. 2.
Metals which were increased in TB patients. Lead, Arsenic, and Copper ions were measured in control (n = 160) and TB (n = 160) patients groups using Flame Atomic Absorption Spectrometry. The results showed that Lead (A), Arsenic (B), and Copper (C) ions are significantly increased in TB patients (P < 0.001, ***).
Fig. 3.
Biometal ratios which were increased in TB patients. Copper/Zinc and Copper/Selenium ratios were measured in control (n = 160) and TB patients (n = 160) groups using Flame Atomic Absorption Spectrometry. The results showed that Copper/Zinc (A) and Copper/Selenium (B) ratios are significantly increased in TB patients (P < 0.001, ***).
Fig. 4.
Serum Calcium did not change in TB patients. Calcium was measured in control (n = 160) and TB (n = 160) patients groups using Flame Atomic Absorption Spectrometry. The results showed that serum Calcium did not significantly changed in TB patients.
4. Discussion
Several studies have been performed regarding trace elements concentration in tuberculosis patients throughout the world. [10], [43] However, due to the lack of such information in Iran, we aimed to evaluate the serum concentrations of metals such as copper, zinc, calcium, selenium, lead, iron and arsenic in patients with active pulmonary tuberculosis in Sistan which is the highest incident area of Iran. [44] According to the results of the present study, the serum levels of zinc were significantly lower in TB patients compared to healthy controls. Similar study was carried out by Taneja et al. They reported that the mean serum zinc concentration in pulmonary TB patients was significantly lower in contrast to the control group. [45] Ciftci et al. conducted a study on the serum concentrations of zinc in TB infected patients in Turkey. Similarly they observed a low zinc concentration in serum of patients. [46] Low serum zinc in TB patients could be due to the redistribution of zinc from plasma to other tissues, reduction of hepatic production of zinc-carrier protein α−2 macro globulin (α−2 M), and increasing the production of metallothionin, a protein that transports zinc to the liver. [46], [47], [48]
In addition to zinc, the serum iron level in TB patients was also low in the present study. Several studies illustrated the lower iron concentration in sera of TB patients compared to the control healthy cases. [12], [13], [17], [19] Boelaert et al. indicated that M. tuberculosis ability in multiplication within host macrophages depends on the available iron. [13] Hence, the iron deficiency that we observed in TB infected patients could be due to the mycobacterial iron consumption. [13], [15], [17], [49] Moreover, we also found that the serum level of selenium in TB patients was significantly lower compared to the healthier controls. A study in China was done by Liu et al. indicated a significantly low levels of molybdenum, zinc, copper, and selenium in the serum of tuberculosis patients. [50] Ramakrishnan et al. showed that serum levels of selenium in patients with active pulmonary tuberculosis are low when compared with healthy cases. [51] Selenium is an important component of anti-oxidative enzymes such as glutathione peroxidase (GPx) which is crucial in the protection of host cells from oxidative damage in inflammatory response and clearing tuberculosis. The antioxidant activity of selenium can alter mycobacterium DNA. To avoid DNA damage, M. tuberculosis has developed effective mechanisms to interrupt the activity of GPx. The impaired GPx can result in selenium deficiency in TB patients. [36]
Unlike zinc, selenium, and iron, significantly high levels of copper were observed in pulmonary TB patients. Similar results also obtained for arsenic and lead in the present study. In a survey conducted in Korea, patients with pulmonary TB had significantly higher serum copper and cobalt than healthy controls, while zinc and selenium concentrations were significantly lower. [43] There is a logical explanation for the association of high copper concentration in TB patient. Decrease in zinc levels, which occurs in TB patient, prevents entrance of the copper to the tissues, and this leads to elevation of serum level of copper. On the other hand, increase in serum level of some metals such as copper or cadmium results in lower absorption of serum iron that is in compliance with our study. [28], [52]
Like copper, most of the patients had high serum levels of lead. In line with this, Afridi et al. indicated significantly higher levels of Pb in scalp hair, blood, and urine samples of TB-HIV co-infected patients, compared with control subjects. [53] Lead can impair total antibody productions, and affects the lymphocytes as well. Although the mechanism of increased serum lead has yet to be elucidated, mycobacterium can escape immune system by using this strategy. [54], [55]
Elevated serum Cu/Zn ratio has been reported in patients with tuberculosis. The serum copper/zinc declined in patients’ blood after anti-tuberculosis treatment. [3] Similarly, we indicated that the ratio of copper/zinc was higher in serum of TB patients compared to that in healthy individuals.
One of the limitations of our study is that we did not evaluate serum cadmium levels of the patients; it might affect serum iron level of the patient. Moreover, food consumption and nutritional status of people in Sistan should be evaluated, as there are several reports that reported malnutrition among healthy population in this region.
5. Conclusion
In conclusion, this study shows that serum levels of different trace elements in pulmonary tuberculosis patients are different compared to the healthy group. We observed low serum concentrations of zinc, iron, selenium, and in contrast, high serum concentration of copper, lead and arsenic in TB patients compared to the healthy cases. Since zinc, iron and selenium are crucial to the immune system function, the low concentrations of these elements can enhance human susceptibility to mycobacterium infection. Hence, Mycobacterium tuberculosis increases the host susceptibility by consuming zinc, iron, and selenium. In contrast, the bacterium escapes the immune system by increasing the serum levels of Pb and Cu as their heir serum concentrations impair human immune system function. However, further investigation should be done to clarify the role of each trace element in the function of host immune responses.
Conflict of interest
Hereby all authors declare that they have no competing interests.
Submission declaration and verification
Hereby authors declare that this paper has not been published before anywhere.
Authorship
D A and A S, M M and A P carried out data gathering, M A has done statistical analysis, A S, H O and Z K prepared the initial drafting of the manuscript. Z S conceived the study, participated in design and coordination of the study. S Gh and N M helped in statistical analysis and drafting the manuscript from beginning to the last point. We also declare that the authors have read and approved the final manuscript
Funding
This work was financially supported by Zabol University of Medical Sciences.
Acknowledgements
We wish to thank the head and staff of the tuberculosis Centers of Zabol, Zahak, Hirmand and Hamoun.
References
- 1.Issoufou I, Sani R, Belliraj L, Ammor FZ, Moussa Ounteini A, Ghalimi J. [Pneumonectomy for tuberculosis destroyed lung: a series of 26 operated cases] Revue Pneumol Clin. 2016;72(5) doi: 10.1016/j.pneumo.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Organization W.H. Tuberculosis. Saudi Med J. 2013;34:1205–1207. [Google Scholar]
- 3.Mohan G., Kulshreshtha S., Sharma P. Zinc and copper in Indian patients of tuberculosis. Biol Trace Elem Res. 2006;111:63–69. doi: 10.1385/BTER:111:1:63. [DOI] [PubMed] [Google Scholar]
- 4.Cavallo F., Gerber M., Pujol H., Marubini E., Decarli A., Richardson S. Zinc and copper in breast cancer. A joint study in northern Italy and southern France. Cancer. 1991;67:738–745. doi: 10.1002/1097-0142(19910201)67:3<738::aid-cncr2820670335>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Hegazy AA, Zaher MM, Abd el-hafez MA, Morsy AA, Saleh RA. Relation between anemia and blood levels of lead, copper, zinc and iron among children. BMC Res Notes. 2010;3:133. doi: 10.1186/1756-0500-3-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voskaki I, Arvanitidou V, Athanasopoulou H, Tzagkaraki A, Tripsianis G, Giannoulia-Karantana A. Serum copper and zinc levels in healthy Greek children and their parents. Biol Trace Elem Res. 2010;134:136–145. doi: 10.1007/s12011-009-8462-2. [DOI] [PubMed] [Google Scholar]
- 7.Schneider JM, Fujii ML, Lamp CL, Lönnerdal B, Zidenberg-Cherr S. The prevalence of low serum zinc and copper levels and dietary habits associated with serum zinc and copper in 12-to 36-month-old children from low-income families at risk for iron deficiency. J Am Dietetic Assoc. 2007;107:1924–1929. doi: 10.1016/j.jada.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Balogh E, Tolnai E, Nagy B,Jr, Nagy B, Balla G, Balla J. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim Biophys Acta. 2016;1862:1640–1649. doi: 10.1016/j.bbadis.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Pfaender S, Fohr K, Lutz AK, Putz S, Achberger K, Linta L. Cellular zinc homeostasis contributes to neuronal differentiation in human induced pluripotent stem cells. Neural Plasticity. 2016;2016 doi: 10.1155/2016/3760702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neyrolles O., Mintz E., Catty P. Zinc and copper toxicity in host defense against pathogens: mycobacterium tuberculosis as a model example of an emerging paradigm. Metal Economy Host-Microbe Interact. 2015;3:1–4. doi: 10.3389/fcimb.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stokowa-Soltys K, Barbosa NA, Kasprowicz A, Wieczorek R, Gaggelli N, Gaggelli E. Studies of viomycin, an anti-tuberculosis antibiotic: copper(ii) coordination, DNA degradation and the impact on delta ribozyme cleavage activity. Dalton Trans. 2016;45:8645–8658. doi: 10.1039/c6dt00245e. (Cambridge, England: 2003) [DOI] [PubMed] [Google Scholar]
- 12.Gangaidzo I.T., Moyo V.M., Mvundura E., Aggrey G., Murphree N.L., Khumalo H. Association of pulmonary tuberculosis with increased dietary iron. J Infectious Dis. 2001;184:936–939. doi: 10.1086/323203. [DOI] [PubMed] [Google Scholar]
- 13.Boelaert JR, Vandecasteele SJ, Appelberg R, Gordeuk VR. The effect of the host's iron status on tuberculosis. J Infectious Dis. 2007;195:1745–1753. doi: 10.1086/518040. [DOI] [PubMed] [Google Scholar]
- 14.Patel SJ, Lewis BE, Long JE, Nambi S, Sassetti CM, Stemmler TL. Fine-tuning of substrate affinity leads to alternative roles of mycobacterium tuberculosis Fe2+-ATPases. J Bioll Chemi. 2016;291:11529–11539. doi: 10.1074/jbc.M116.718239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mwandumba HC, Russell DG, Nyirenda MH, Anderson J, White SA, Molyneux ME. Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J Immunol. 2004;172:4592–4598. doi: 10.4049/jimmunol.172.7.4592. [DOI] [PubMed] [Google Scholar]
- 16.Venketaraman V, Rodgers T, Linares R, Reilly N, Swaminathan S, Hom D. Glutathione and growth inhibition of Mycobacterium tuberculosis in healthy and HIV infected subjects. AIDS Res Therapy. 2006;3:1. doi: 10.1186/1742-6405-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermid JM, Prentice A.M. Clinical science (London, England: 1979; 2006. Iron and infection: effects of host iron status and the iron-regulatory genes haptoglobin and NRAMP1 (SLC11A1) on host-pathogen interactions in tuberculosis and HIV. 110: 503-524. [DOI] [PubMed] [Google Scholar]
- 18.Kerkhoff AD, Meintjes G, Opie J, Vogt M, Jhilmeet N, Wood R. Anaemia in patients with HIV-associated TB: relative contributions of anaemia of chronic disease and iron deficiency. Int J Tuberculosis Lung Dis. 2016;20:193–201. doi: 10.5588/ijtld.15.0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratledge C. Iron, mycobacteria and tuberculosis. Tuberculosis. 2004;84:110–130. doi: 10.1016/j.tube.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Meneghetti F, Villa S, Gelain A, Barlocco D, Chiarelli LR, Pasca MR. Curr Med Chem; 2016. Iron acquisition pathways as targets for antitubercular drugs. [DOI] [PubMed] [Google Scholar]
- 21.Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I. Mycobacterial P 1-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe. 2011;10:248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stensland I, Kim JC, Bowring B, Collins AM, Mansfield JP, Pluske JR. A comparison of diets supplemented with a feed additive containing organic acids, cinnamaldehyde and a permeabilizing complex, or zinc oxide, on post-weaning diarrhoea, selected bacterial populations, blood measures and performance in weaned pigs experimentally infected with enterotoxigenic E. coli. Animals. 2015;5:1147–1168. doi: 10.3390/ani5040403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramakrishnan K, Shenbagarathai R, Kavitha K, Uma A, Balasubramaniam R, Thirumalaikolundusubramanian P. Serum zinc and albumin levels in pulmonary tuberculosis patients with and without HIV. Jpn J Infectious Dis. 2008;61:202. [PubMed] [Google Scholar]
- 24.Bacelo AC, Ramalho A, Brasil PE, Cople-Rodrigues Cdos S, Georg I, Paiva E. Nutritional supplementation is a necessary complement to dietary counseling among tuberculosis and tuberculosis-HIV patients. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osman D, Piergentili C, Chen J, Chakrabarti B, Foster AW, Lurie-Luke E. Generating a metal-responsive transcriptional regulator to test what confers metal sensing in cells. J Biol Chem. 2015;290:19806–19822. doi: 10.1074/jbc.M115.663427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chauhan S, Kumar A, Singhal A, Tyagi JS, Krishna Prasad H. CmtR, a cadmium-sensing ArsR-SmtB repressor, cooperatively interacts with multiple operator sites to autorepress its transcription in Mycobacterium tuberculosis. FEBS J. 2009;276:3428–3439. doi: 10.1111/j.1742-4658.2009.07066.x. [DOI] [PubMed] [Google Scholar]
- 27.Grammer T.B., Kleber M.E., Silbernagel G., Pilz S., Scharnagl H., Lerchbaum E. Copper, ceruloplasmin, and long-term cardiovascular and total mortality (the Ludwigshafen Risk and Cardiovascular Health Study) Free Radic Res. 2014;48:706–715. doi: 10.3109/10715762.2014.901510. [DOI] [PubMed] [Google Scholar]
- 28.Liu T., Ramesh A., Ma Z., Ward S.K, Zhang L., George G.N. CsoR is a novel mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol. 2007;3:60–68. doi: 10.1038/nchembio844. [DOI] [PubMed] [Google Scholar]
- 29.Shah S., Dalecki A.G., Malalasekera A.P., Crawford C.L., Michalek S.M., Kutsch O. 8-Hydroxyquinolines are boosting agents of copper-related toxicity in mycobacterium tuberculosis. 2016;60(10):5765–5776. doi: 10.1128/AAC.00325-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith A.H., Marshall G., Yuan Y., Liaw J., Ferreccio C., Steinmaus C. Evidence from Chile that arsenic in drinking water may increase mortality from pulmonary tuberculosis. Am J Epidemiol. 2010;93:1–6. doi: 10.1093/aje/kwq383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amare B., Moges B., Mulu A., Yifru S., Kassu A. Quadruple burden of HIV/AIDS, tuberculosis, chronic intestinal parasitoses, and multiple micronutrient deficiency in ethiopia: a summary of available findings. BioMed Res Int. 2015;2015 doi: 10.1155/2015/598605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eick F, Maleta K, Govasmark E, Duttaroy A, Bjune A. Food intake of selenium and sulphur amino acids in tuberculosis patients and healthy adults in Malawi [Short communication] Int J Tuberculosis Lung dis. 2009;13:1313–1315. [PubMed] [Google Scholar]
- 33.Seyedrezazadeh E., Ostadrahimi A., Mahboob S., Assadi Y., Ghaemmagami J., Pourmogaddam M. Effect of vitamin E and selenium supplementation on oxidative stress status in pulmonary tuberculosis patients. Respirology. 2008;13:294–298. doi: 10.1111/j.1440-1843.2007.01200.x. [DOI] [PubMed] [Google Scholar]
- 34.Mehto S., Antony C., Khan N., Arya R., Selvakumar A., Tiwari B.K. Mycobacterium tuberculosis and human immunodeficiency virus type 1 cooperatively modulate macrophage apoptosis via toll like receptor 2 and calcium homeostasis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin Y., Liu L., Zhang S., He R., Wu Y., Chen G. Cadmium exposure to murine macrophages decreases their inflammatory responses and increases their oxidative stress. Chemosphere. 2016;144:168–175. doi: 10.1016/j.chemosphere.2015.08.084. [DOI] [PubMed] [Google Scholar]
- 36.Wu G., Yi Y. Effects of dietary heavy metals on the immune and antioxidant systems of Galleria mellonella larvae. Comparative Biochem Physiol Toxicol Pharmacol. 2015;167:131–139. doi: 10.1016/j.cbpc.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Jayawardena U.A., Ratnasooriya W.D., Wickramasinghe D.D., Udagama P.V. Heavy metal mediated innate immune responses of the Indian green frog, Euphlyctis hexadactylus (Anura: Ranidae): cellular profiles and associated Th1 skewed cytokine response. Sci Total Env. 2016;566-567:1194–1204. doi: 10.1016/j.scitotenv.2016.05.171. [DOI] [PubMed] [Google Scholar]
- 38.Rohini K, Bhat S, Srikumar P, Kumar AM. Assessment of serum calcium and phosphorus in pulmonary tuberculosis patients before, during and after chemotherapy. Indian J Clin Biochem. 2014;29:377–381. doi: 10.1007/s12291-013-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eshghi P, Alavi S, Ghavami S, Rashidi A. Growth impairment in beta-thalassemia major: the role of trace element deficiency and other potential factors. J Pediatr Hematol Oncol. 2007;29:5–8. doi: 10.1097/MPH.0b013e31802d74f3. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Teo KC. Determination of cadmium, copper, lead and zinc in water samples by flame atomic absorption spectrometry after cloud point extraction. Analyt. Chim. Acta. 2001;450:215–222. doi: 10.1039/b008717n. [DOI] [PubMed] [Google Scholar]
- 41.Barbosa VMP, Barbosa AF, Bettini J, Luccas PO, Figueiredo EC. Direct extraction of lead (II) from untreated human blood serum using restricted access carbon nanotubes and its determination by atomic absorption spectrometry. Talanta. 2016;147:478–484. doi: 10.1016/j.talanta.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 42.Zhong W-S, Ren T, Zhao L-J. Determination of Pb (Lead), Cd (Cadmium), Cr (Chromium), Cu (Copper), and Ni (Nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. J Food Drug Anal. 2016;24:46–55. doi: 10.1016/j.jfda.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi R, Kim H-T, Lim Y, Kim M-J, Kwon OJ, Jeon K. Serum concentrations of trace elements in patients with tuberculosis and its association with treatment outcome. Nutrients. 2015;7:5969–5981. doi: 10.3390/nu7075263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pourakbari B, Mamishi S, Mohammadzadeh M, Mahmoudi S. First-line anti-tubercular drug resistance of mycobacterium tuberculosis in IRAN: a systematic review. Frontiers Microbiol. 2016;7:1139. doi: 10.3389/fmicb.2016.01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taneja D. Observations on serum zinc in patients of pulmonary tuberculosis. J Indian Med Assoc. 1990;88(275):280–281. [PubMed] [Google Scholar]
- 46.Ciftci TU, Ciftci B, Yis O, Guney Y, Bilgihan A, Ogretensoy M. Changes in serum selenium, copper, zinc levels and cu/zn ratio in patients with pulmonary tuberculosis during therapy. Biol Trace Elem Res. 2003;95:65–71. doi: 10.1385/BTER:95:1:65. [DOI] [PubMed] [Google Scholar]
- 47.Kassu A., Yabutani T., Mahmud Z., Mohammad A., Nguyen N., Huong B. Alterations in serum levels of trace elements in tuberculosis and HIV infections. Eur J Clin Nutr. 2006;60:580–586. doi: 10.1038/sj.ejcn.1602352. [DOI] [PubMed] [Google Scholar]
- 48.Koyanagi A, Kuffo D, Gresely L, Shenkin A, Cuevas LE. Relationships between serum concentrations of C-reactive protein and micronutrients, in patients with tuberculosis. Ann Tropical Med Parasitol. 2004;98:391–399. doi: 10.1179/000349804225003424. [DOI] [PubMed] [Google Scholar]
- 49.Agarwal R., Tiwari V., Agarwal R., Gupta N. To evaluate the presence of anemia in patients with pulmonary tuberculosis at a tertiary health care centre. PARIPEX-Indian Res. 2016;5:75–76. [Google Scholar]
- 50.Liu X., Ding L, Wang Y, Yang Y. [Determination of trace elements in serum of tuberculosis patients] Wei Sheng Yan Jiu. 2000;29:395–396. [PubMed] [Google Scholar]
- 51.Ramakrishnan K, Sharma SP, Shenbagarathai R, Kavitha K, Thirumalaikolundusubramanian P. Serum selenium levels in pulmonary tuberculosis levels with and without HIV/AIDS. Retrovirology. 2009;6:1. [Google Scholar]
- 52.Wolschendorf F, Ackart D, Shrestha TB, Hascall-Dove L, Nolan S, Lamichhane G. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci. 2011;108:1621–1626. doi: 10.1073/pnas.1009261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Afridi HI, Kazi TG, Kazi N, Kandhro GA, Shah AQ, Baig JA. Evaluation of arsenic, cadmium, lead, nickel, and zinc in biological samples (scalp hair, blood, and urine) of tuberculosis and diarrhea male human immunodeficiency virus patients. Clinl Lab. 2011;57:867–878. [PubMed] [Google Scholar]
- 54.Mishra K. Lead exposure and its impact on immune system: a review. Toxicol Vitro. 2009;23:969–972. doi: 10.1016/j.tiv.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Skoczyńska A, Poreba R, Sieradzki A, Andrzejak R, Sieradzka U. [The impact of lead and cadmium on the immune system] Medycyna Pracy. 2001;53:259–264. [PubMed] [Google Scholar]