Figure 7.
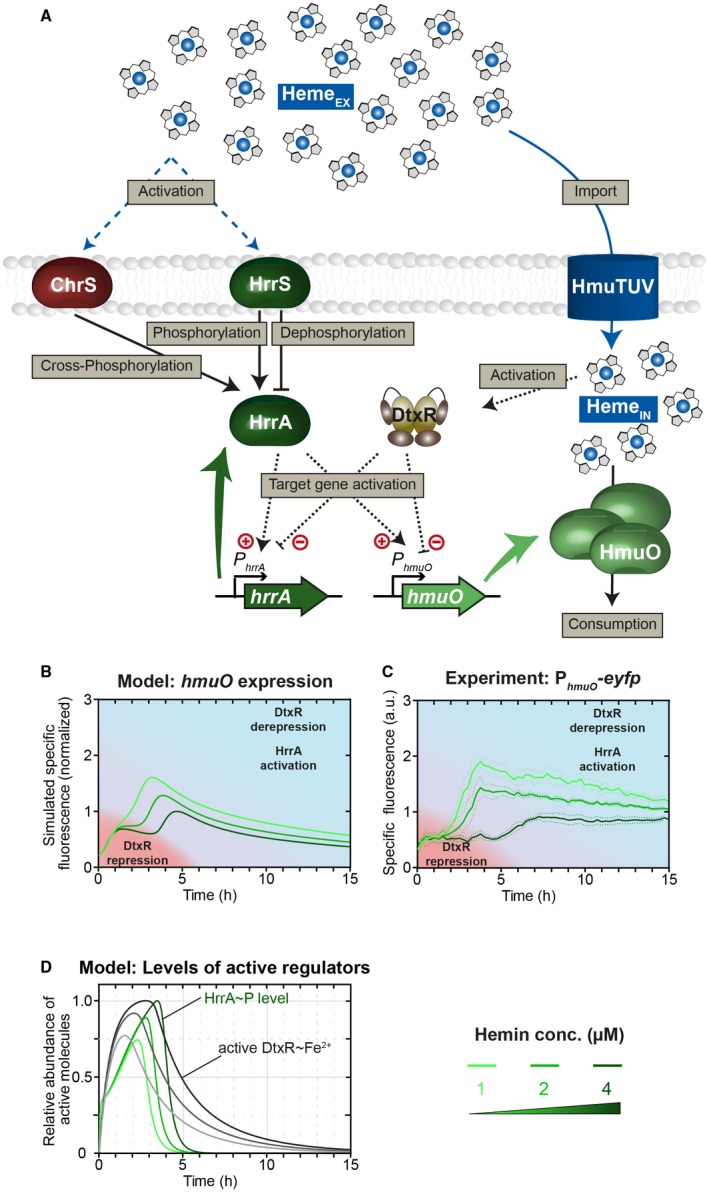
Information on iron availability is integrated into the HrrSA‐regulated utilization module hmuO via DtxR. A. The mathematical model of the heme utilization module in C. glutamicum shares many basic assumptions with the model of heme detoxification (for description see Fig. 3). Here, heme consumption is assumed to be supported by the heme oxygenase HmuO, whose production is regulated by the phosphorylated response regulator HrrA~P and the iron repressor DtxR. The activation of DtxR is expected to be influenced by internal heme levels. DtxR repression and HrrA activation shape the delayed response of the heme utilization module hmuO. B. The PhmuO promoter is activated by the phosphorylated response regulator HrrA~P after a significant time delay of 3–5 h. Higher heme concentrations lead to a prolonged delay and a lower hmuO expression in general. C. The mathematical model of the heme utilization network in C. glutamicum could reproduce this behavior and gave an explanation regarding. D. The temporal dynamics of both regulators on PhmuO. Levels of the activated iron repressor DtxR increase immediately after addition of heme and repress the promotor activation of PhmuO, proportional to stimulus strength. However, HrrA~P levels increase with a short time delay in response to the stimulus and activate the promotor to a certain extend at the beginning and with increasing intensity upon DtxR dissociation [Colour figure can be viewed at wileyonlinelibrary.com]
