Summary
Background
Topical ionic contraviral therapy (ICVT) with digoxin and furosemide inhibits the potassium influx on which DNA viruses rely for replication. Therefore, ICVT was hypothesized to be a potential novel treatment for cutaneous warts.
Objectives
To assess the clinical efficacy, safety and tolerability of ICVT in adults with cutaneous warts. The secondary objective was to gain insight into the underlying working mechanism of ICVT.
Methods
Treatment with ICVT was assessed for efficacy, safety and tolerability in a single‐ centre, randomized, double‐blind, placebo‐controlled phase IIA trial. Eighty adult patients with at least two cutaneous warts (plantar or common) were randomized to one of four treatments: digoxin + furosemide (0·125%), digoxin (0·125%), furosemide (0·125%) or placebo. The gel was administered once daily for 42 consecutive days. Predefined statistical analysis was performed with a mixed‐model ancova. The trial was registered at ClinicalTrials.gov with number NCT02333643.
Results
Wart size and human papillomavirus (HPV) load reduction was achieved in all active treatment groups. A statistically significant reduction in wart diameter of all treated warts was shown in the digoxin + furosemide treatment group vs. placebo (−3·0 mm, 95% confidence interval −4·9 to −1·1, P = 0·002). There was a statistically significant reduction in the HPV load of all treated warts in the digoxin + furosemide group vs. placebo (−94%, 95% confidence interval −100 to −19, P = 0·03). With wart size reduction, histologically and immunohistochemically defined viral characteristics disappeared from partial and total responding warts.
Conclusions
This study demonstrates the proof of concept for the efficacy of topical ICVT in adults with cutaneous warts.
Short abstract
What's already known about this topic?
Cutaneous warts are caused by the human papillomavirus (HPV).
Ionic contraviral therapy (ICVT) might be a potential treatment for cutaneous warts.
A previous phase I/II open‐label study demonstrated the safety and efficacy of ICVT.
What does this study add?
Proof of concept for the efficacy of topical ICVT in adults with cutaneous warts.
Topical ICVT demonstrates a favourable safety profile, with the effects most pronounced when it is combined in a formulation for common warts.
Wart size reduction was related to HPV load reduction measured by quantitative polymerase chain reaction (qPCR) in swabs.
qPCR is a valuable disease biomarker for drug development in cutaneous warts.
https://doi.org/10.1111/bjd.17803 available online
Cutaneous warts, or verrucae, are a common benign skin condition with an estimated prevalence of 3–13% in the general population in the Western world.1 Most people are affected by cutaneous warts, either plantar warts (located on the foot soles) or common warts (mostly located on the hands or dorsal feet), at some point in their life.1, 2, 3, 4
Although cutaneous warts are benign and usually resolve spontaneously,5 they cause both physical and psychosocial discomfort.6 Many patients use a variety of wart‐removing products.6, 7, 8 Efficacy rates of common treatments are approximately 39% for cryotherapy, 24% for salicylic acid and 46% for monochloroacetic acid, whereas spontaneous regression rates are around 16%.7, 9, 10, 11 As current treatments such as cryotherapy and monochloroacetic acid often have side‐effects (e.g. pain, erythema and burning sensation)12 and low efficacy rates, there is a need for therapies with a greater efficacy and minimal side‐effects.13, 14, 15
Cutaneous warts are caused by the human papillomavirus (HPV). The great majority (> 80%) of verrucae in the general population are related to HPVs 1, 2, 27 and 57.16, 17, 18, 19, 20, 21 It is well known that papillomaviruses are dependent of the milieu of the infected host cell for proliferation.22, 23 More specifically, it has been shown that DNA viruses, such as HPV, rely on potassium ion influx for replication.24 The cardiac glycoside digoxin and loop diuretic furosemide both inhibit K+ influx by interacting with the cell‐membrane ion cotransporters Na+/K+‐ATPase and Na‐K‐Cl. These two compounds may therefore be valuable for the treatment of HPV‐induced diseases, such as cutaneous warts. In 2006, an in vitro study found that the inhibitory effect on DNA replication was most potent when digoxin and furosemide were combined. This new approach with two well‐known, established drugs, described as ionic contraviral therapy (ICVT), is suggested to be most effective via local application.25
A previous phase I/II open‐label study recently demonstrated the safety and efficacy of ICVT in a group of 12 healthy patients with common warts.26 The aim of the current proof‐of‐concept study was to assess the clinical efficacy, safety and tolerability of ICVT in adults with cutaneous warts in a single‐centre, randomized, double‐blind, placebo‐controlled phase IIA trial. The secondary objective was to gain insight into the underlying working mechanism of ICVT.
Patients and methods
Study design, participants and randomization
A randomized, double‐blind, placebo‐controlled, parallel‐group, single‐centre phase II trial was conducted. The Declaration of Helsinki was the guiding principle for trial execution, and the study was approved by the independent medical ethics committee ‘Medisch Ethische Toetsingscommissie van de Stichting Beoordeling Ethiek Biomedisch Onderzoek’ (Assen, the Netherlands) prior to any procedure. Patients were included if they were healthy (other than the skin condition), aged ≥ 18 years and had at least two (nonsubungual, nongenital and nonfacial) common or plantar warts with a diameter ≥ 3 mm, diagnosed by a dermatologist and after giving written informed consent. A maximum of five warts per subtype were followed during the study. Patients were excluded if they had been exposed to wart‐removing products within 30–60 days prior to enrolment, depending on the treatment. For women of childbearing age, effective contraception was required during study execution and ≥ 90 days afterwards. The study consisted of a screening phase (weeks −4 to 0), a treatment phase (weeks 0–6) and a follow‐up phase (weeks 6–14), as shown in Figure 1.
Figure 1.
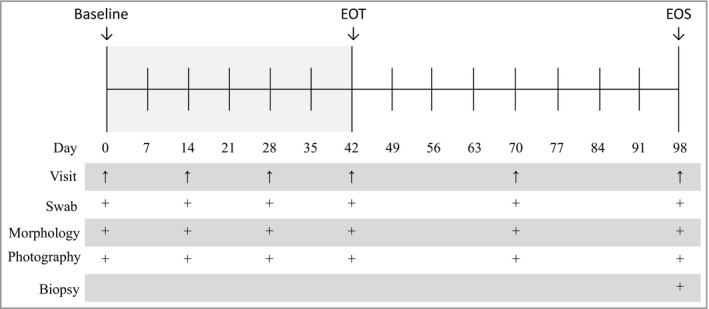
Study design. The treatment phase lasted 42 days with study visits at days 0, 14, 28 and 42. The follow‐up phase lasted for 56 days with study visits at days 70 and 98. At all visits the following assessments were performed of all warts: wart size measurement, wart morphology, photography and swab. At day 98, a biopsy was performed of the primary and untreated warts. EOT, end of treatment; EOS, end of study.
Patients were randomized 1 : 1 : 1 : 1 in blocks of four to receive one of the four treatment regimens: digoxin + furosemide (0·125%, w/w) digoxin (0·125% w/w), furosemide (0·125% w/w) or vehicle, which served as placebo with an identical appearance. Randomization was predefined and performed in SAS (SAS Institute Inc., Cary, NC, U.S.A.) by an independent statistician, and patient numbers were sequentially allocated by chronological enrolment. The patients, study personnel and investigators were blinded for allocated treatment throughout the study. At baseline, all warts were numbered by a blinded independent clinical staff member: for common warts starting from 1 with a maximum of 5 and for plantar warts starting from 6 with a maximum of 10. Wart number 1 or 6 was selected as the untreated wart (n = 80) and the other warts were selected as treated warts. Of the treated warts, one wart per patient was selected as the primary wart (biopsy wart, n = 80) using a randomly generated number in SAS drawn by an independent statistician.
Study site
The study was conducted from December 2014 to August 2015 at the Center for Human Drug Research, Leiden, the Netherlands.
Study procedures
The primary objective was to investigate the clinical efficacy of ICVT by analysing wart size reduction and viral load in primary warts in the four treatment groups. Wart size reduction was assessed in diameter and height (mm) by a digital vernier caliper (0–150 mm) (Conrad Electronic Benelux B.V., Oldenzaal, the Netherlands). Wart clearance (defined as 100% reduction) was assessed by a dermatological subinvestigator. Viral load was measured with use of skin swabs.26 In addition, two biopsies of the primary wart and the untreated reference wart were taken at the end of study (EOS). The HSL‐PCR/MPG assay (LMNX kit HSL‐PCR; Labo Bio‐medical Products, Rijswijk, the Netherlands) enables the simultaneous identification of 23 wart‐associated HPV types from the alpha (HPV 2, 3, 7, 10, 27, 28, 29, 40, 43, 57, 77, 91 and 94), gamma (HPV 4, 48, 50, 60, 65, 88 and 95), mu (HPV1 and 63) and nu genera (HPV41).16, 27 Viral load was determined for all swabs and biopsy samples of primary warts that were positive for HPV 1, 2, 27 or 57 by quantitative polymerase chain reaction (qPCR).
The secondary objective was to gain insight into the underlying working mechanism of ICVT. Therefore, wart morphology was assessed to confirm or reject the hypothesis that wart size reduction could be predicted by the morphological aspects of all warts in this study. Standardized photographs of the primary wart were taken and wart morphology was assessed using the CWARTS diagnostic tool.28, 29 Complete responders were defined as showing a reduction of 100% in size, partial responders a reduction of 25–100% and nonresponders < 25% reduction at the EOS compared with baseline. A subset of 20 warts was chosen based on response (complete, partial or nonresponder) for analysis by histopathology and immunohistochemistry (IHC) in order to confirm or reject the hypothesis that wart size reduction can be predicted by viral characteristics, Ki‐67 (cell proliferation) and HPV E4 (a marker of a productive infection) patterns. Viral characteristics (histopathology), Ki‐67 (clone MIB‐1; Agilent, Santa Clara, CA, U.S.A.) and HPV E4 patterns (SILgrade‐E4‐1 kit containing XR‐E4‐1 monoclonal antibody; Labo Bio‐medical Products) were assessed by two blinded reviewers and without prior knowledge of the responder or HPV status. All analyses were independently performed by two reviewers, except for the Ki67 analysis, which was discussed during microscopy.
Safety and tolerability were monitored by tracking of adverse events; performing physical examination; measuring vital signs; 12‐lead electrocardiograms; laboratory tests (haematology, chemistry, coagulation and urinalysis) and systemic therapeutic drug monitoring for systemic exposure of digoxin at multiple time points throughout the study. Treatment adherence was measured by monitoring all daily‐dose administrations via a validated mobile e‐diary app. After application of the gel, trial patients took a photo of all warts with use of the mobile e‐diary.
Statistics
A sample size of 20 patients per treatment group was estimated based on the analysis of primary warts to provide > 90% power to demonstrate the superiority of digoxin and/or furosemide over placebo with a difference in means of 31·6 mm3, assuming that the common SD is 30, using a two‐group t‐test with a 0·05 two‐sided significance level.26 All efficacy and pharmacodynamic end points were analysed in the intention‐to‐treat population, with a mixed model using treatment, time and treatment by time as fixed factors and patient as a random factor. The predefined primary analyses to investigate the clinical efficacy of ICVT were performed for primary warts only. The predefined secondary analysis to gain insight into the underlying working mechanism of ICVT was based on all treated warts, and within patient was added as a random factor to the model. All statistical tests were two tailed with an α‐level of 0·05. A two‐sided Fisher's exact test and a two‐sided Wilcoxon exact rank test were used to analyse wart clearance. Correlation between qPCR in swab samples and biopsies was investigated using a linear regression model with patient as a random factor.
Results
Patients
In total 114 otherwise healthy patients with cutaneous warts were screened, of whom 81 (71%) were enrolled in the trial; one withdrew before randomization (Figs 1, 2). All patients (n = 80) completed the study and there were no treatment discontinuations or early withdrawals. The baseline demographic and disease characteristics were comparable in all four treatment groups (Table 1).
Figure 2.
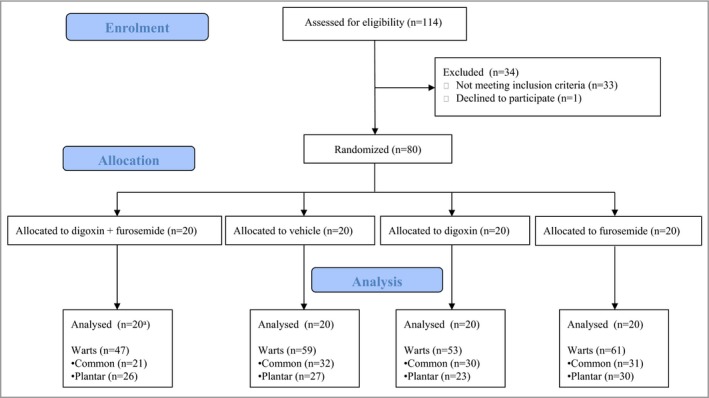
Flowchart of the study of all patients and warts. In total 114 otherwise healthy patients with cutaneous warts were screened, of whom 81 (71%) were enrolled in the trial; one withdrew before randomization. Of the 80 remaining patients, 20 were randomly assigned to each of the four treatment groups: digoxin + furosemide, digoxin, furosemide or placebo, all to be locally applied in gels. All patients (n = 80) completed the study and there were no treatment discontinuations or early withdrawals. aIn the digoxin + furosemide group the pharmacodynamics measurements of the primary wart of one patient were excluded.
Table 1.
Patient characteristics, n = 20 in each group
| Characteristics | Digoxin + furosemidea | Digoxin | Furosemide | Placebo | Total |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 23·8 ± 7·9 | 30 ± 13·5 | 23·5 ± 5·5 | 26·1 ± 12·7 | 25·8 ± 10·6 |
| Sex, n (%) | |||||
| Male | 6 (30) | 11 (55) | 7 (35) | 7 (35) | 31 (39) |
| Female | 14 (70) | 9 (45) | 13 (65) | 13 (65) | 49 (61) |
| Time since diagnosis (years), mean | 5·3 | 7·6 | 6·9 | 4·9 | 6·2 |
| Total number of warts | 47 | 53 | 61 | 59 | 220 |
| Warts per patients, mean | 2·4 | 2·7 | 3·1 | 3 | 2·8 |
| Patients with common warts, n (%) | 9 (45) | 10 (50) | 10 (50) | 10 (50) | 39 (49) |
| Total common warts, n (%) | 21 (45) | 30 (57) | 31 (51) | 32 (54) | 114 (52) |
| Treated common warts, n (%) | 12 (57) | 19 (63) | 21 (68) | 21 (66) | 73 (64) |
| Patients with plantar warts, n (%) | 11 (55) | 9 (45) | 10 (50) | 9 (45) | 39 (49) |
| Total plantar warts, n (%) | 26 (55) | 23 (43) | 30 (49) | 27 (46) | 106 (48) |
| Treated plantar warts, n (%) | 15 (58) | 13 (57) | 20 (67) | 17 (63) | 65 (61) |
| Patients with both common and plantar warts, n (%) | 0 | 1 (5) | 0 | 1 (5) | 2 (3) |
| Diameter of warts (mm), mean | 6·6 | 6·4 | 6·4 | 6·5 | 6·5 |
| Diameter of primary wart (mm), mean | 6·02 | 6·56 | 6·47 | 6·45 | 6·38 |
| HPV type in the primary wart | |||||
| HPV1 | 0 | 0 | 0 | 0 | 0 |
| HPV2 | 5 | 4 | 3 | 6 | 18 |
| HPV27 | 6 | 10 | 10 | 3 | 29 |
| HPV57 | 6 | 2 | 3 | 6 | 17 |
| Otherb | 2 | 4 | 4 | 5 | 15 |
| Any previous treatment, n (%) | 16 (80) | 17 (85) | 14 (70) | 15 (75) | 62 (78) |
| Cryotherapy, n (%) | 12 (60) | 16 (80) | 12 (60) | 14 (70) | 54 (68) |
| Cimetidine, n (%) | 0 | 1 (5) | 0 | 0 | 1 (1) |
| Electrocoagulation, n (%) | 0 | 1 (5) | 0 | 0 | 1 (1) |
| Fluorouracil, n (%) | 0 | 1 (5) | 0 | 0 | 1 (1) |
| Monochloroacetic acid, salicylic acid or trichloroacetic acid, n (%) | 7 (35) | 8 (40) | 9 (45) | 6 (30) | 30 (38) |
| Surgery, n (%) | 1 (5) | 2 (10) | 0 | 0 | 3 (4) |
HPV, human papillomavirus. aIn the digoxin + furosemide group the pharmacodynamics measurements of the primary wart of one patient were excluded. bOther: HPV3, HPV4 and HPV10.
Treatment adherence
Seventy‐eight of the 80 patients (98%) applied the gel once daily for more than 35 consecutive days, and only sporadically did patients not comply with the daily treatment regimen. Most patients applied a dose within the range of 5–30 mg per wart per day. However, the mean amount of study medication applied per wart per day was highly variable (range 2·9–118 mg).
Wart size reduction
Figure 3(a) shows a reduction in primary wart diameter measured by caliper from baseline to EOS in all active treatment groups. A statistically significant effect (P < 0·05) was found in the digoxin + furosemide group vs. placebo [−2·5 mm, 95% confidence interval (CI) −4·9 to −0·1, P = 0·04], while the two other treatment groups (digoxin vs. placebo and furosemide vs. placebo) showed no statistically significant differences (−1·5 mm, 95% CI −3·9 to 0·9, P = 0·21; and −1·1 mm, 95% CI −3·4 to 1·3, P = 0·38, respectively). Changes in diameter were most pronounced after the end of treatment, as shown in Figure 3(a). In the analysis of all treated warts (n = 139) a statistically significant wart size reduction measured by caliper was observed between each active treatment group and placebo, as shown in Figure 3(b): digoxin + furosemide vs. placebo, −3·0 mm, 95% CI −4·9 to −1·1, P = 0·002; digoxin vs. placebo, −1·9 mm, 95% CI −3·7 to −0·2, P = 0·03; furosemide vs. placebo, −2·1 mm; 95% CI −3·8 to −0·4, P = 0·01.
Figure 3.
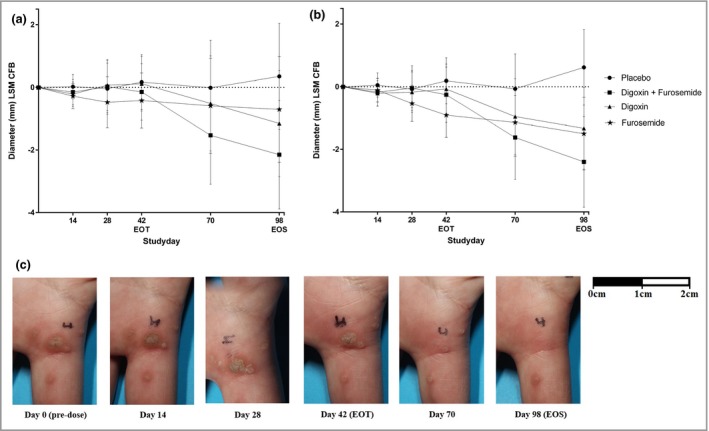
Change from baseline (CFB) least squares mean (LSM) of the diameter of primary warts (a) and all treated warts (b), and photographic assessment of a common wart of patient 6 (digoxin + furosemide) (c). (a) Analysis of the primary end point for the intention‐to‐treat population (n = 79) was performed using a mixed model with treatment, time and treatment by time as fixed factors and patient as a random factor. All statistical tests were two tailed with an α‐level of 0·05. The results showed a statistically significant reduction of wart size in the digoxin + furosemide group vs. placebo [−2·5 mm, 95% confidence interval (CI) −4·9 to −0·1, P = 0·04). Single treatment groups (digoxin vs. placebo and furosemide vs. placebo) showed no statistically significant effects (−1·5 mm, 95% CI −3·9 to 0·9, P = 0·21; and −1·1 mm, 95% CI −3·4 to 1·3, P = 0·38, respectively). Changes in diameter were most pronounced after the end of treatment (EOT). (b) In the analysis of all treated warts (n = 139) a statistically significant wart size reduction was observed between each active treatment group and placebo (digoxin + furosemide vs. placebo, −3·0 mm, 95% CI −4·9 to −1·1, P = 0·002; digoxin vs. placebo, −1·9 mm, 95% CI −3·7 to −0·2, P = 0·03; furosemide vs. placebo, −2·1 mm, 95% CI −3·8 to −0·4, P = 0·01). (c) Photographic assessment of a treated wart in the digoxin + furosemide group. EOS, end of study.
Wart clearance
At the EOS, primary warts (n = 80) showed comparable clearance rates in all active treatment groups: three of 19 (16%) in the digoxin + furosemide group, three of 20 (15%) in the digoxin group and three of 20 (15%) in the furosemide group. In contrast, no clearance was observed in the placebo‐treated group (n = 20). A two‐sided Fisher's exact test revealed no statistically significant differences when the active treatment groups were compared with the placebo group. In Table 2 for all 3 treatment groups comparable clearance rates are shown in all treated warts, i.e. the primary target wart and the other treated warts. Table S1 (see Supporting Information) shows the rates of clearance observed in treated common warts (24–27%) and treated plantar warts (8–15%) at the EOS. When including all warts with a reduction of ≥ 90% diameter, the highest response rate was seen in common warts treated with digoxin + furosemide (n = 5) at the EOS, with a response rate of 45%. In Figure 3(c) an example of a photographic assessment of a treated wart in the digoxin + furosemide group is shown.
Table 2.
Clearance of all warts per patient at the end of the study
| Characteristics | Digoxin + furosemide (n = 19)a | Digoxin (n = 20) | Furosemide (n = 20) | Placebo (n = 20) |
|---|---|---|---|---|
| Wart clearanceb (P‐value treatment vs. placebo) | 0·11 | 0·23 | 0·11 | – |
| All warts cleared, n (%) | 2 (11) | 2 (10) | 2 (10) | 0 |
| At least one wart, but not all warts cleared, n (%) | 1 (5) | 1 (5) | 2 (10) | 0 |
| No clearance, n (%) | 16 (84) | 17 (85) | 16 (80) | 20 (100) |
aIn the digoxin + furosemide group the pharmacodynamics measurements of the primary wart of one patient were excluded. bClearance defined as 100% reduction.
Viral load
At baseline, 200 of the 219 warts (91%; one missing sample) were positive for DNA from the 23 tested HPV types. HPV27 was most prevalent (38%), followed by HPV57 (26%) and HPV2 (24%). Of the 219 warts, 186 (85%) were positive for one of the HPV types for which viral load testing was available (HPV1, 2, 27, 57). No statistical differences were found when comparing the HPV load of primary warts (n = 79) in swabs from baseline to the EOS in the treatment groups with those in the placebo group, as shown in Figure 4(a): digoxin + furosemide, −8%, 95% CI −96 to 1952, P = 0·96; digoxin, −6·3%, 95% CI −96 to 2086, P = 0·97; furosemide, 80%, 95% CI −92 to 3966, P = 0·71). However, when comparing the viral load change of HPV from baseline to the EOS in the swabs of all treated warts (n = 139), there was a statistically significant reduction of viral load, but only in the digoxin + furosemide group vs. placebo (−94%, 95% CI −100 to −19, P = 0·03) (Fig. 4b). In biopsies, no statistically significant differences in HPV load were seen in the treatment groups vs. placebo. There was a significant correlation (P < 0·001) between viral load in swabs and biopsies at the EOS (Fig. 4c). We observed a significant correlation (P = 0·001) between wart size reduction and reduction in HPV load (data not shown).
Figure 4.
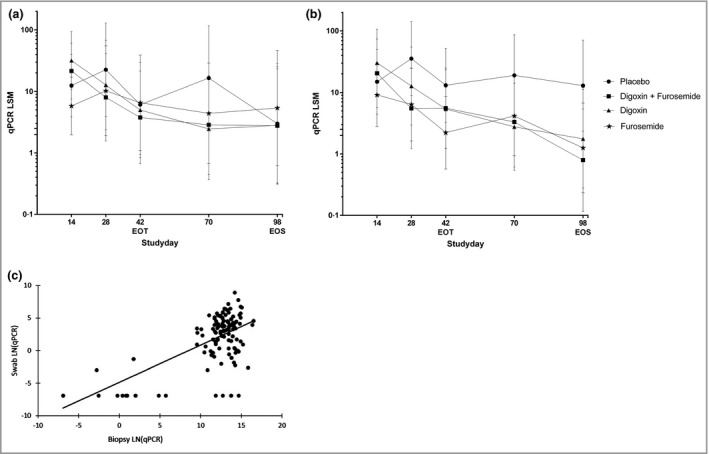
Human papillomavirus (HPV) viral load in swabs depicted as the percentage change from baseline least squares mean (LSM) of primary warts (a) and all treated warts (b), and correlation of HPV viral load in swabs vs. biopsy at the end of study (EOS) (c). (a) Analysis of primary warts (n = 79) was performed using a mixed model with treatment, time and treatment by time as fixed factors and patient as a random factor. All statistical tests were two tailed with an α‐level of 0·05. No statistical differences were found when comparing the HPV load of primary warts in swabs from baseline to EOS in the treatment groups with those in the placebo group [digoxin + furosemide, −8%, 95% confidence interval (CI) −96 to 1952, P = 0·96; digoxin, −6·3%, 95% CI −96 to 2086, P = 0·97; furosemide, 80%, 95% CI −92 to 3966, P = 0·71]. (b) The viral load change of HPV from baseline to the EOS in the swabs of all treated warts (n = 139) was statistically significant only in the digoxin + furosemide group vs. placebo (−94%, 95% CI −100 to −19, P = 0·03). (c) Correlation between quantitative polymerase chain reaction (qPCR) in swab samples and biopsies was investigated using a linear regression model with patient as a random factor. There was a significant correlation (P < 0·001) between the viral load in swabs and biopsies at the EOS. The line depicts the linear correlation: viral load swab = −4·8 + 0·56 × viral load biopsy. EOT, end of treatment.
Response analyses
Regarding wart elevation, a significantly decreased wart diameter was observed at the EOS after 6 weeks of treatment with the combination treatment digoxin + furosemide compared with placebo (−5·2 mm, 95% CI −8·6 to −1·8, P = 0·003). The morphological aspects callus and smooth/rough wart did not show any differences in prediction of wart size reduction (Table 3).
Table 3.
Wart morphology in relation to wart size in the digoxin + furosemide treatment group
| Wart diameter (mm) | ||
|---|---|---|
| Difference (95% CI)a | P‐value | |
| Callus | −1·71 (−5·12 to 1·70) | 0·32 |
| Present (n = 42) | ||
| Absent (n = 37) | ||
| Capillary thrombosis | −2·51 (−6·15 to 1·13) | 0·17 |
| Present (n = 45) | ||
| Absent (n = 34) | ||
| Level | −5·21 (−8·60 to −1·82) | 0·0031 |
| Elevation (n = 44) | ||
| Flat (n = 35) | ||
| Aspect | −1·86 (−5·85 to 2·13) | 0·34 |
| Smooth (n = 17) | ||
| Rough (n = 62) | ||
CI, confidence interval. aDifference of the mean diameter as measured by caliper.
In Table 4 a summary of the responder analysis is given, based on nine responder warts (three complete and six partial) and 11 nonresponders. The individual data are available in Table S2 (see Supporting Information). Haematoxylin and eosin staining showed changes characteristic of viral infection in biopsies from nonresponder warts in contrast to the biopsies from complete and partial responder warts. In the IHC of the nonresponders, Ki‐67 was positive suprabasal (scattering) in all biopsies, compared with a basal Ki‐67 pattern in all complete responders (three of three, 100%) and five of six (83%) partial responders (Table 4). Staining of the HPV E4 protein, indicative of a productive HPV infection, was positive in all nonresponders and was related to a high HPV load in EOS biopsies and swabs (Table 4). Concordantly, in all complete and partial responders the E4 staining was negative. The mean viral load in biopsies and swabs at the EOS was lower in the complete and partial responders than in the nonresponders. Figure 5 illustrates examples of the haematoxylin and eosin staining of a classical verruca vulgaris and verruca plana, showing typical viral characteristics.
Table 4.
Response analyses per responder group: haematoxylin and eosin immunohistochemical staining of biopsies and viral load in biopsies and swabs
| Response | Complete (n = 3) | Partial (n = 6) | None (n = 11) |
|---|---|---|---|
| Swab baseline | |||
| HPV positive | TND (1/3)a | HPV2 (2/6) | HPV2 (3/11) |
| HPV3 (1/3) | HPV27 (2/6) | HPV27 (4/11) | |
| HPV57 (1/3) | HPV57 (2/6) | HPV57 (4/11) | |
| Mean log10 copies by PCR | 5·6b | 4·7 | 4·9 |
| Biopsy at the end of study | |||
| Viral characteristics present | 0/3 | 0/6 | 11/11 |
| Positive test for E4, Ki‐67 and HPV | E4 (0/3) | E4 (0/6) | E4 (11/11) |
| Ki‐67 (3/3)c | Ki‐67 (5/6),c (1/6)d | Ki‐67 (11/11)d | |
| HPV2 (1/3) | HPV2 (1/6) | HPV2 (4/11) | |
| HPV27 (1/6) | HPV27 (4/11) | ||
| HPV57 (3/6) | HPV57 (3/11) | ||
| Mean log10 copies by PCR | 2·9e | 3·8e | 8·8 |
| Swab at the end of study | |||
| HPV positive | HPV (0/3) | HPV2 (2/6) | HPV27 (4/11) |
| HPV57 (2/6) | HPV2 (4/11) | ||
| HPV57 (3/11) | |||
| Mean log10 copies by PCR | 0e | 1·1e , f | 4·3 |
The data are presented as n/N unless stated otherwise. HPV, human papillomavirus; PCR, polymerase chain reaction. aTarget not detected (TND) for the 23 HPV types included in the broad‐spectrum genotyping assay. bSamples of patients without HPV DNA detected or HPV3 at baseline were not further tested for viral load and therefore are not included in the mean. cBasal staining, restricted to the basal layer. dScattered staining. eSamples not tested are considered as zero. fSamples where the target is not detected are considered as zero.
Figure 5.
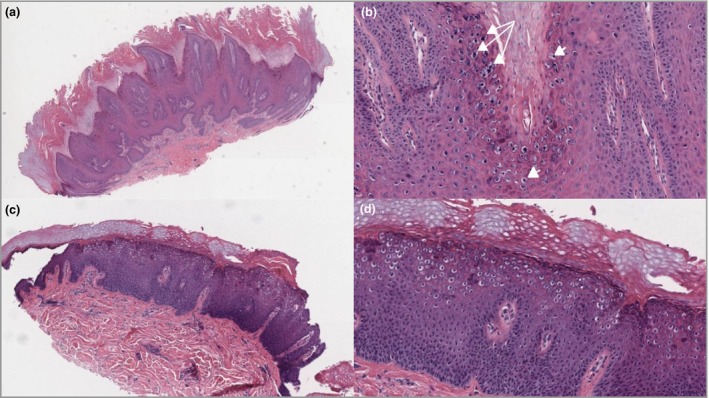
Histological representative cases of classical cutaneous viral warts. (a) Verruca vulgaris: haematoxylin and eosin (H&E) low‐power view (original magnification × 50) with architectural characteristic inturning of the elongated rete ridges, epidermal hyperplasia, papillomatosis, hypergranulosis, hyperkeratosis and columns of parakeratosis. (b) Verruca vulgaris: H&E, detail view (× 200). Note koilocytes (arrowheads) and coarse granuloma (arrows) mostly in the top layers (stratum granulosum). (c) H&E low‐power view (× 50) of verruca plana with epidermal hyperplasia, hypergranulosis, hyperkeratosis and koilocytes in the middle and upper layers. (d) Verruca plana: H&E, detail view (× 100). Note the absence of papillomatosis, parakeratosis and coarse granuloma.
Safety
No treatment‐related study discontinuations occurred. The adverse event profile was comparable in all treatment groups. Nasopharyngitis, headache and influenza‐like illness were the most frequently occurring mild and self‐limiting treatment‐emergent adverse events (Table S3; see Supporting Information). No clinically relevant changes in vital signs or laboratory assessments were observed. The digoxin values measured for therapeutic drug monitoring were all below the limit of quantification (300 pg mL−1).
Discussion
This study demonstrates clear and statistically significant pharmacodynamic effects of topical ICVT on common and plantar warts, with a favourable safety profile. Both lesion reduction and clearance rates indicate pharmacological activity and demonstrate proof of concept of ICVT in adults with cutaneous warts.
The effects of ICVT were slightly more pronounced in patients with common warts. This is in accordance with previous studies, wherein evident differences between response to treatment of common and plantar warts were reported.7, 30 The increased treatment resistance of plantar warts was previously described and seems to be mainly due to callus formation resulting in a decrease in the cutaneous permeability of a drug.28
The efficacy rates of the most commonly used treatments are estimated to be around 39% for cryotherapy, 24% for salicylic acid and 46% for monochloroacetic acid. In the current study, the ICVT efficacy rates were estimated to be comparable with those reported in the literature, around 45% in common warts. However, it should be noted that the current trial consisted of patients with treatment‐resistant warts that had been present for a long time (mean time of onset 4·9–7·6 years in the treatment groups). It can therefore be anticipated that ICVT might have shown higher efficacy rates in patients with more recently developed warts.
Interestingly, wart size clearance and reduction in diameter both occurred predominantly after the end of treatment. One explanation might be that ICVT interferes with the HPV life cycle,22 which results firstly in a reduction of HPV load and thereafter reduction in wart size. It looks like the disappearance of signs of HPV infection precedes the actual vanishing of the wart. This is supported by the fact that E4 staining, indicative of a productive infection, in the response analysis showed that partially cleared warts were in viral regression, showing fewer E4 signals and fewer papillary patterns. Another explanation could be reservoir forming of ICVT in the hyperkeratotic layer that slowly releases the drug into the lesion, thereby resulting in a delayed and prolonged response. Studies with a longer follow‐up period and without the biopsy intervention at the EOS have to be considered to understand better the effectiveness of ICVT in both the mono‐active and dual‐active forms.
Warts without application of the research gel in the active treatment groups reduced in size, in contrast to those in the placebo group, which suggests that this reduction was not due to spontaneous regression. The observed clearance might be explained by distant effects of the gel; for example, increased activation of the immune system might have led to activity in untreated distant warts. Cardiac glycosides such as digoxin are known to influence the immune response at multiple levels,31 thus digoxin in the formulation might be held responsible for this. This distant clearance concept is also known from another topical compound, imiquimod. Patients with psoriasis treated with imiquimod can locally develop total‐body psoriasis exacerbations during treatment based on distant skin immune system activation by imiquimod.32, 33, 34
The distribution of HPV types in warts in this study was similar to that found in common and plantar warts in the literature,21 except for HPV1. This can logically be explained by the study sample, containing adults, whereas HPV1 infections are more prevalent among children with warts present for < 6 months.21
Skin swabs have frequently been used to determine the HPV status of patients in a research setting, but not yet in relation to antiviral treatment monitoring.35, 36 Wart swabs are ideal for sampling in order to determine viral load, as the gold‐standard HPV status determination (biopsy) has several disadvantages such as the burden for the patient, the practical difficulty of taking multiple biopsies from a single small lesion, and the potential study bias caused by the curative effect of taking a biopsy.37 The current study showed that viral load determined in swabs correlated with viral load determined from biopsies of the same wart. These data confirm the correlation previously reported by van der Kolk et al., but now in a larger sample set, warranting the continued use of this marker in clinical studies.26
The outcomes from the microscopic and IHC analyses of the biopsies at the EOS correspond with those from the viral load analysis. Biopsies and swabs of the complete and partial responders had a lower viral load or were HPV negative, which corresponds with loss of changes in the epithelium characteristic of viral infection, absence of E4 staining and basal Ki‐67 staining, whereas the nonresponders had high viral loads in swab and biopsies. Haematoxylin and eosin staining of the biopsies showed signs of changes related to viral infection, E4 staining and scattered Ki‐67 staining. The HPV E4 protein disrupts the keratin filament network and inhibits formation of the cornified envelope. Detection of E4 is indicative of a productive viral infection.22, 38 Ki‐67 is a biomarker for cell proliferation, and in normal epithelium the Ki‐67 signals are restricted to the basal layer. By reactive change, the Ki‐67 positivity is also observed in the other layers of the epithelium (scattered staining).39 From this we can conclude that there is a clear correlation between the histopathological diagnoses, presence of E4 and Ki‐67 pattern and HPV load.
Determining the morphological aspects of the warts could be useful to predict wart size reduction based on the results of the current study. In clinical practice this might be helpful to provide insight into the morphological characteristics when deciding about the most effective and personalized treatment. Current options for therapy all have high rates of side‐effects including pain and irritation at the application site, blistering and scarring.7, 13 Such local irritations were not observed in the current trial.
In conclusion, our findings clearly show proof of concept of topical ICVT for cutaneous warts, with the most pronounced effects of digoxin and furosemide seen when they were combined in a formulation for common warts. A treatment period of 42 days was well tolerated and led to significant wart size reduction and occasionally clearance. As hypothesized, wart size reduction was related to HPV load reduction, measured by qPCR in the swab, proving that this swab method can be a valuable, noninvasive disease biomarker for drug development in cutaneous warts. As clinical outcomes such as clearance of lesion sites often require long‐term treatment and follow‐up, we indicate the efficacy shown in the current study as proof of concept of ICVT in cutaneous warts. Further investigations to evaluate total clearance and recurrence rates after longer treatment and follow‐up periods are recommended.
Supporting information
Table S1 Clearance per wart at the end of the study.
Table S2 Response analyses.
Table S3 Summary of treatment‐emergent adverse events reported in more than one patient.
Acknowledgments
The authors would like to thank all of the patients for their participation in the clinical trial, and Karen Broekhuizen for the thorough support in medical writing.
Funding sources Cutanea Life Science, Wayne, PA, U.S.A.
Conflicts of interest None to declare.
https://doi.org/10.1111/bjd.17803 available online
References
- 1. Beliaeva TL. The population incidence of warts. Vestn Dermatol Venerol 1990; 2:55–8 (in Russian). [PubMed] [Google Scholar]
- 2. van Haalen FM, Bruggink SC, Gussekloo J et al Warts in primary schoolchildren: prevalence and relation with environmental factors. Br J Dermatol 2009; 161:148–52. [DOI] [PubMed] [Google Scholar]
- 3. Kyriakis K, Pagana G, Michailides C et al Lifetime prevalence fluctuations of common and plane viral warts. J Eur Acad Dermatol Venereol 2007; 21:260–2. [DOI] [PubMed] [Google Scholar]
- 4. Kilkenny M, Merlin K, Young R, Marks R. The prevalence of common skin conditions in Australian school students: 1. Common, plane and plantar viral warts. Br J Dermatol 1998; 138:840–5. [DOI] [PubMed] [Google Scholar]
- 5. Massing AM, Epstein WL. Natural history of warts. A two‐year study. Arch Dermatol 1963; 87:306–10. [DOI] [PubMed] [Google Scholar]
- 6. Ciconte A, Campbell J, Tabrizi S et al Warts are not merely blemishes on the skin: a study on the morbidity associated with having viral cutaneous warts. Australas J Dermatol 2003; 44:169–73. [DOI] [PubMed] [Google Scholar]
- 7. Kwok CS, Gibbs S, Bennett C et al. Topical treatments for cutaneous warts. Cochrane Database Syst Rev 2012; 9:CD001781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruggink SC, Eekhof JA, Egberts PF et al Natural course of cutaneous warts among primary schoolchildren: a prospective cohort study. Ann Fam Med 2013; 11:437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bruggink SC, Gussekloo J, Berger MY et al Cryotherapy with liquid nitrogen versus topical salicylic acid application for cutaneous warts in primary care: randomized controlled trial. CMAJ 2010; 182:1624–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bruggink SC, Gussekloo J, Egberts PF et al Monochloroacetic acid application is an effective alternative to cryotherapy for common and plantar warts in primary care: a randomized controlled trial. J Invest Dermatol 2015; 135:1261–7. [DOI] [PubMed] [Google Scholar]
- 11. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta‐analysis and pooled analysis of randomized controlled trials. Br J Dermatol 2011; 165:233–46. [DOI] [PubMed] [Google Scholar]
- 12. Ockenfels HM. Therapeutic management of cutaneous and genital warts. J Dtsch Dermatol Ges 2016; 14:892–9. [DOI] [PubMed] [Google Scholar]
- 13. Sterling JC, Gibbs S, Haque Hussain SS et al British Association of Dermatologists’ guidelines for the management of cutaneous warts 2014. Br J Dermatol 2014; 171:696–712. [DOI] [PubMed] [Google Scholar]
- 14. Sterling JC, Handfield‐Jones S, Hudson PM. Guidelines for the management of cutaneous warts. Br J Dermatol 2001; 144:4–11. [DOI] [PubMed] [Google Scholar]
- 15. Bruggink SC, Waagmeester SC, Gussekloo J et al Current choices in the treatment of cutaneous warts: a survey among Dutch GP. Fam Pract 2010; 27:549–53. [DOI] [PubMed] [Google Scholar]
- 16. de Koning MN, Ter SJ, Eekhof JA et al Evaluation of a novel broad‐spectrum PCR‐multiplex genotyping assay for identification of cutaneous wart‐associated human papillomavirus types. J Clin Microbiol 2010; 48:1706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan SY, Chew SH, Egawa K et al Phylogenetic analysis of the human papillomavirus type 2 (HPV‐2), HPV‐27, and HPV‐57 group, which is associated with common warts. Virology 1997; 239:296–302. [DOI] [PubMed] [Google Scholar]
- 18. Hagiwara K, Uezato H, Arakaki H et al A genotype distribution of human papillomaviruses detected by polymerase chain reaction and direct sequencing analysis in a large sample of common warts in Japan. J Med Virol 2005; 77:107–12. [DOI] [PubMed] [Google Scholar]
- 19. Porro AM, Alchorne MM, Mota GR et al Detection and typing of human papillomavirus in cutaneous warts of patients infected with human immunodeficiency virus type 1. Br J Dermatol 2003; 149:1192–9. [DOI] [PubMed] [Google Scholar]
- 20. Chen SL, Tsao YP, Lee JW et al Characterization and analysis of human papillomaviruses of skin warts. Arch Dermatol Res 1993; 285:460–5. [DOI] [PubMed] [Google Scholar]
- 21. Bruggink SC, de Koning MN, Gussekloo J et al Cutaneous wart‐associated HPV types: prevalence and relation with patient characteristics. J Clin Virol 2012; 55:250–5. [DOI] [PubMed] [Google Scholar]
- 22. Doorbar J. The papillomavirus life cycle. J Clin Virol 2005; 32(Suppl. 1):S7–15. [DOI] [PubMed] [Google Scholar]
- 23. Doorbar J, Quint W, Banks L et al The biology and life‐cycle of human papillomaviruses. Vaccine 2012; 30 (Suppl. 5):F55–70. [DOI] [PubMed] [Google Scholar]
- 24. Hartley CE, Buchan A, Randall S et al The effects of lithium and potassium on macromolecular synthesis in herpes simplex virus‐infected cells. J Gen Virol 1993; 74:1519–25. [DOI] [PubMed] [Google Scholar]
- 25. Hartley C, Hartley M, Pardoe I, Knight A. Ionic contra‐viral therapy (ICVT); a new approach to the treatment of DNA virus infections. Arch Virol 2006; 151:2495–501. [DOI] [PubMed] [Google Scholar]
- 26. van der Kolk T, Dillingh MR, Rijneveld R et al Topical ionic contra viral therapy comprised of digoxin and furosemide as a potential novel treatment approach for common warts. J Eur Acad Dermatol Venereol 2017; 31:2088–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Koning MN, Khoe LV, Eekhof JA et al Lesional HPV types of cutaneous warts can be reliably identified by surface swabs. J Clin Virol 2011; 52:84–7. [DOI] [PubMed] [Google Scholar]
- 28. Hogendoorn GK, Bruggink SC, de Koning MNC et al Morphological characteristics and human papillomavirus genotype predict the treatment response in cutaneous warts. Br J Dermatol 2018; 178:253–60. [DOI] [PubMed] [Google Scholar]
- 29. Hogendoorn GK, Bruggink SC, Hermans KE et al Developing and validating the Cutaneous WARTS (CWARTS) diagnostic tool: a novel clinical assessment and classification system for cutaneous warts. Br J Dermatol 2018; 178:527–34. [DOI] [PubMed] [Google Scholar]
- 30. Bruggink SC, Gussekloo J, de Koning MN et al HPV type in plantar warts influences natural course and treatment response: secondary analysis of a randomised controlled trial. J Clin Virol 2013; 57:227–32. [DOI] [PubMed] [Google Scholar]
- 31. Kepp O, Menger L, Vacchelli E et al Anticancer activity of cardiac glycosides: at the frontier between cell‐autonomous and immunological effects. Oncoimmunology 2012; 1:1640–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel U, Mark NM, Machler BC, Levine VJ. Imiquimod 5% cream induced psoriasis: a case report, summary of the literature and mechanism. Br J Dermatol 2011; 164:670–2. [DOI] [PubMed] [Google Scholar]
- 33. Chakrabarty AK, Mraz S, Geisse JK, Anderson NJ. Aphthous ulcers associated with imiquimod and the treatment of actinic cheilitis. J Am Acad Dermatol 2005; 52 (2 Suppl. 1):35–7. [DOI] [PubMed] [Google Scholar]
- 34. Maronas‐Jimenez L, Morales‐Raya C, Burillo‐Martinez S et al Aphthous vulvar ulcers: a paradoxal adverse effect at distance of topical imiquimod? Eur J Obstet Gynecol Reprod Biol 2016; 198:156–7. [DOI] [PubMed] [Google Scholar]
- 35. Hazard K, Karlsson A, Andersson K et al Cutaneous human papillomaviruses persist on healthy skin. J Invest Dermatol 2007; 127:116–19. [DOI] [PubMed] [Google Scholar]
- 36. Weissenborn SJ, De Koning MN, Wieland U et al Intrafamilial transmission and family‐specific spectra of cutaneous betapapillomaviruses. J Virol 2009; 83:811–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petry KU, Horn J, Luyten A, Mikolajczyk RT. Punch biopsies shorten time to clearance of high‐risk human papillomavirus infections of the uterine cervix. BMC Cancer 2018; 18:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doorbar J. The E4 protein; structure, function and patterns of expression. Virology 2013; 445:80–98. [DOI] [PubMed] [Google Scholar]
- 39. Chow LT, Broker TR. Human papillomavirus infections: warts or cancer? Cold Spring Harb Perspect Biol 2013; 5:a012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Clearance per wart at the end of the study.
Table S2 Response analyses.
Table S3 Summary of treatment‐emergent adverse events reported in more than one patient.


