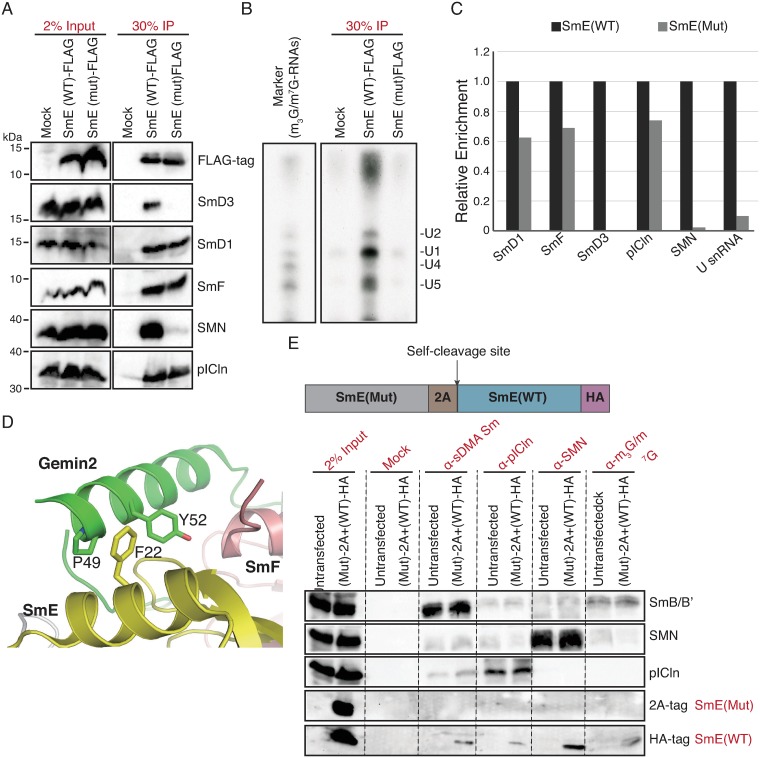
Fig 2. The missense mutation impairs the biogenesis of spliceosomal U snRNPs during Sm core assembly.
(A-E), The NSM mutation in SmE impairs its interaction with U snRNP assembly machinery and incorporation into U snRNPs. (A), Anti-FLAG immunoprecipitation after transient transfection in HEK293T cells and western blotting analysis for co-precipitated U snRNP intermediates. Mock immunoprecipitations were performed with untransfected lysates. (B), 3’-end labeling of co-precipitated RNA and autoradiography. RNA immunoprecipitated using the H20 antibody against m3G/m7G cap of U snRNAs was used as reference. (C), Quantification of the data shown in A and B from two independent biological replicates, with black bars representing wild type and gray bars representing mutant SmE. (D), Predicted structural model for interference of the SmE mutation in its interaction with Gemin2, based on the PDB structure 4V98. (E), Immunoprecipitation using antibodies specific to Sm proteins, SMN, pICn and U snRNA cap, with lysates from HEK293T cells transfected with dual expression plasmid encoding 2A-tagged mutant SmE and HA-tagged wild type SmE and western blotting to analyze the integration of the wild type and mutant SmE into U snRNP biogenesis pathway.