Figure 5.
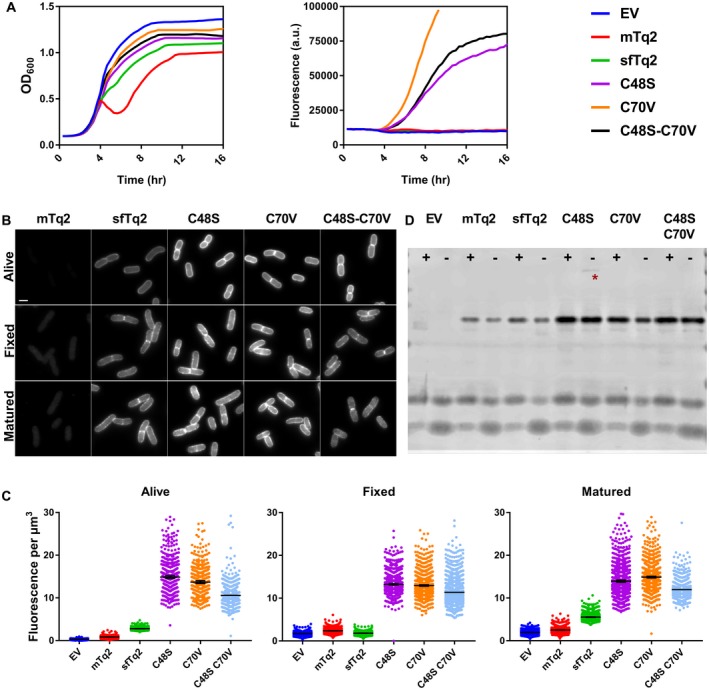
Cysteine‐replaced sfTq2 variants perform better in the periplasm. A. Periplasmic expression of sfTq2‐PBP5 variants in LMC500 grown in rich medium at 37°C at relatively non‐toxic induction conditions results in large differences in cyan fluorescence. The C70V variant performs better than C48S and the double cysteine mutant version. B. Microscopy of LMC500 grown in rich medium and induced with 15 µM IPTG shows strong fluorescence for the sfTq2‐PBP5 variants in the periplasm. For comparison, the greyscale of all photographs are the same (80–7000) and the scale bar represents 2 µm. C. Quantification of the images confirms bright fluorescence signals from the single C48S and C70V sfTq2 variants. Differences in fluorescence with the prolonged plate reader induction suggest that folding difficulties may still be a problem. The error bars at the mean represent the 95% confidence interval. The number of cells measured were between: Alive 500–1000, Fixed 1000–1500 and Matured 1000–2000 except for EV‐Alive n = 327 and C48S‐C70V‐Fixed n = 775. D. mTq2 is produced at similar levels as sfTq2 but does not fold properly; cysteine mutants of sfTq2 are produced at higher levels. Anti‐GFP immunoblotting of the corresponding samples shows that the fusion proteins are intact and that C48S has a minor propensity to form higher order complexes (asterisk). The + and signs, respectively, indicate the presence or absence of the reducing agent DTT in the sample.
