Summary
Obesity is a worldwide growing problem. When confronted with obesity, many health care providers focus on direct treatment of the consequences of adiposity. We plead for adequate diagnostics first, followed by an individualized treatment. We provide experience‐based and evidence‐based practical recommendations (illustrated by clinical examples), to detect potential underlying diseases and contributing factors. Adult patients consulting a doctor for weight gain or obesity should first be clinically assessed for underlying diseases, such as monogenetic or syndromic obesity, hypothyroidism, (cyclic) Cushing syndrome, polycystic ovarian syndrome (PCOS), hypogonadism, growth hormone deficiency, and hypothalamic obesity. The most important alarm symptoms for genetic obesity are early onset obesity, dysmorphic features/congenital malformations with or without intellectual deficit, behavioral problems, hyperphagia, and/or striking family history. Importantly, also common contributing factors to weight gain should be investigated, including medication (mainly psychiatric drugs, (local) corticosteroids, insulin, and specific β‐adrenergic receptor blockers), sleeping habits and quality, crash diets and yoyo‐effect, smoking cessation, and alcoholism. Other associated conditions include mental factors such as chronic stress or binge‐eating disorder and depression.Identifying and optimizing the underlying diseases, contributing factors, and other associated conditions may not only result in more effective and personalized treatment but could also reduce the social stigma for patients with obesity.
Keywords: Diagnostics, genetic obesity, hormones, medication, secondary causes
1. INTRODUCTION
Obesity (body mass index [BMI] ≥30.0 kg/m2) is a chronic disease1 that is a worldwide growing problem.2 In 2015, over 603 million adults had obesity, and 4.0 million deaths were yearly accountable to a high BMI.3 When facing a patient with obesity, most clinicians focus on treating the associated comorbidities and/or simply recommend weight loss. The diagnostic phase, which is typically used when assessing other clinical problems, is often overlooked in obesity. Whereas in other clinical problems, such as hypertension, clinicians are alert to consider a wide variety of secondary causes.4 For obesity, a diagnostic phase to detect underlying diseases or other factors that hinder weight loss is recommended by some5 but not all clinical practice guidelines. The European guideline recommends a diagnostic phase; however, specific examples of diseases or factors that may contribute to obesity are lacking.5 Others do not mention the necessity to identify underlying contributing factors.6, 7 This paper aims to help clinicians to deepen the diagnostic phase with a comprehensive overview of possible contributors to weight gain for an individual.
Currently, on a societal level, it is widely believed that obesity is simply the consequence of overconsuming unhealthy foods and lack of exercise. However, on an individual level, there are many other contributing factors or underlying diseases, which are often not identified and may prove significantly associated with weight gain and barriers to weight loss. We suggest that clinicians should first detect and address underlying diseases and contributing factors, before starting obesity treatment. Besides lifestyle‐related factors, other factors include hormonal and genetic abnormalities, mental and socio‐cultural factors, and side effects of medications. Identifying these underlying factors may lead to more personalized treatment strategies, can also increase patients' understanding of their obesity, and reduce their social stigma.
1.1. Clinical presentation
1.1.1. Clinical Case A
A 66‐year‐old man, known with type 2 diabetes mellitus and eczema, was referred because of obesity. Since the last decade, he became increasingly heavier, with a rapid weight gain of 30 kg in the last year. He noticed red striae under his armpits and suffered from mood swings and decreased libido. A detailed past medication list showed several episodes of using clobetason cream, triamcinolone, desoximetason, and one short course of prednisone in the last 8 years. Moreover, in the last year, he had used large amounts of mometason cream on his whole back and arms because of severe eczema. We saw a man weighing 168 kg (BMI 59.5 kg/m2) with pronounced abdominal obesity with purple striae, hematomas, and severe non‐pitting ankle edema. Laboratory results are shown in Table 1. We concluded that there was severe obesity, with multiple contributing causes: an exogenous Cushing syndrome due to the use of large amounts of dermal corticosteroids, late‐onset hypogonadism (which can be a consequence of obesity but also poses an opportunity for treatment8), and non‐pitting lymphedema. The subclinical hypothyroidism was considered a consequence of obesity.9, 10 A multidisciplinary strategy comprised gradual tapering of topical corticosteroids and lymphedema compression therapy by his dermatologist, testosterone supplementation, and an exercise program. After 2 years, he had lost 35 kg, his clinical condition improved, and testosterone and TSH levels had normalized. This case shows the importance of a combined approach taking into account all factors that contribute to obesity and counteract weight loss.
Table 1.
Laboratory results patient 1
| Value | Reference Value | |
|---|---|---|
| TSH | 6.030 mU/L | 0.4‐4.3 mU/L |
| FT4 | 15.4 pmol/L | 11‐25 pmol/L |
| Urinary cortisol first measurement | 39 nmol/24 h | 5‐133 nmol/24 h |
| Urinary cortisol second measurement | 48 nmol/24 h | |
| Testosterone | 5.05 nmol/L | 10‐30 nmol/L |
| SHBG | 40 nmol/L | 10‐70 nmol/L |
| Calculated free testosterone | 0.0839 nmol/L | >220 pmol/La |
In the presence of symptoms.93
1.1.2. Clinical Case B
A 48‐year‐old woman was referred because of severe obesity with type 2 diabetes, with no long‐term effect of previous diets. Her overweight started around her first year of life, and later she had been treated in a mental health institute for her eating behavior. Since she was a baby, she always had an increased appetite. Several family members, mostly from her mother's side, had obesity. Interestingly, she reported that even during the Dutch extreme famine of 1945, her maternal grandfather had obesity. We saw a woman with red hair, weighing 117 kg (BMI 41.5 kg/m2). No other physical abnormalities were found.
We suspected a monogenetic cause of her obesity, because of the young age of onset, hyperphagia, red hair, and family history. Diagnostic screening of 52 obesity associated genes revealed two mutations in the melanocortin 4 receptor gene (MC4R) gene, of whom one is known to be pathogenic (c.[105C > A], p.[Tyr35*]), whereas the other is a variant of unknown significance (VUS). Her daughter, who had obesity since the age of three, had inherited both MC4R mutations. Her father, who developed obesity at an older age, had no MC4R mutations.
In patients with pathogenic MC4R mutations long‐term weight maintenance is difficult to achieve.11 The response to bariatric surgery seems to be positive; however, long‐term results are still being investigated.12 Additionally, new pharmacological treatment options are arising that target MC4R.13 Recently, effective pharmacological treatment for another type of monogenetic obesity, caused by pathogenic POMC gene mutations, has become available.14 It is therefore relevant to identify underlying monogenic causes of obesity, which also may reduce the patient's obesity stigma.
2. METHODS
Due to the comprehensive nature of the subject, we selected subtopics based on clinical experience. For each subtopic, we searched databases such as the Cochrane library (February 2018) and MEDLINE library for relevant articles. We preferably selected publications of the last 5 years. Older publications were included if commonly referenced, highly regarded, or relevant to the topic. Reference lists of relevant identified articles were also searched.
3. ASSESSMENT OF OBESITY—CLINICAL HISTORY
Weight gain occurs when the energy homeostasis is chronically “out of balance.” This occurs either due to changes in total energy intake or in total energy expenditure, the latter being the sum of a person's resting energy expenditure plus a person's thermogenesis during activities.15 A clinical consult addressing obesity should therefore focus on what causes this excess and what maintains it. Examples to guide the clinical approach of identifying underlying causes and contributing factors, roughly grouped in lifestyle‐related factors, medication, (neuro‐)endocrine factors, genetic factors, and mental factors, are shown in Figure 1.
Figure 1.
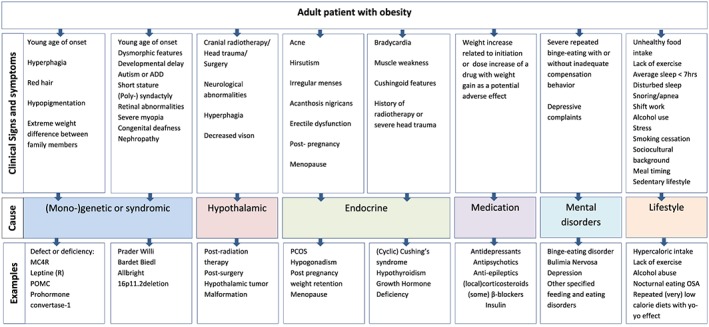
Recognizing underlying causes of obesity in adults. ADD, attention deficit disorder; PCOS, polycystic ovarian syndrome; MC4R, melanocortin 4 receptor; POMC, proopiomelanocortin; PPI, proton pump inhibitors; OC, oral contraceptives; OSA, obstructive sleep apnea; OSFED, other specified feeding and eating disorders [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.1. Lifestyle‐related factors involved in weight gain
Globally, the obesity pandemic is largely the consequence of increased energy consumption.16 However, in individual patients, there may be several reasons why a person has an increased caloric intake or decreased energy expenditure, which may even be modifiable. Often, there is a complex interplay of multiple social, psychological, and biological factors altogether resulting in excess energy intake.
For example, in some cultures, exorbitant amounts of food are associated with hospitality. Also, some patients may be unable to prioritize weight management in light of financial problems, relationship issues, or other circumstances requesting their attention. On an individual level, patients may overeat because they experience increased hunger or appetite. For example, this occurs in patients who have been on very low calorie‐diets without exercise or behavioral therapy, often referred to as the “yo‐yo‐effect.” The weight regain that follows may be associated with altered “hunger hormones” (eg, ghrelin) and satiety hormones' (eg, leptin and peptide YY [PYY]) that can remain altered even a year after ending the diet.17 Some individuals may overeat as a coping strategy for other, psychological factors such as emotions.18Next, a decreased quantity or quality of sleep can induce weight gain.19 This may lead to a desire for high caloric food,20 imbalance of appetite hormones (eg, ghrelin and leptin),21 as well as increased hypothalamic–pituitary–adrenal‐axis reactivity22 yielding higher cortisol levels which may also enhance obesity.23, 24 Circadian misalignment, such as in shift work, is associated with a decreased daily energy expenditure and increased caloric intake.25 , 26 As for sleep quality, obstructive sleep apnea (OSA) is especially notable as it seems to have a bidirectional relation with obesity. OSA occurs more frequently in obesity, but the sleep disturbances belonging to it may again promote weight gain enhancing behavioral, metabolic, and/or hormonal.27 It is not fully clear whether OSA treatment has an effect on body weight.28, 29, 30
When there is no notable change in energy intake, the problem may be altered energy expenditure, which can be either the result of decreased activity or decreased resting metabolism.
Recently, several possible influencers of resting metabolism have been identified. For example, weight loss itself can already affect the total energy expenditure, as this decreases more than is expected from the change in body composition only. This may be due to adaptive thermogenesis, that may hinder further weight loss.31 Also, an important contributor to a decreased metabolism is a sedentary lifestyle.32 Interesting associations have been found for options to interrupt the continuous sitting, eg, by intermittent standing, moving, or fidgeting, also called nonexercise activity thermogenesis (NEAT).33 Another possible determinant and future target regarding resting energy expenditure is the activity of brown adipose tissue (BAT).34 BAT, which was found to be also present in adult humans and basically burns calories into heat, can be stimulated by physical activity or cold exposure. This has been shown to result in a significant decrease in fat mass, with maintenance of lean mass.35 Nicotine can also stimulate BAT,36 possibly explaining the subtle weight gain that patients who quit smoking experience (together with increased appetite and psychological factors). Importantly, this modest weight gain does usually not outweigh the beneficial effects of smoking cessation. Education and guidance may limit the weight gain when patients quit smoking.37
3.2. Medication
Medication can affect the energy homeostasis mainly by promoting hunger or by decreasing resting metabolism. In our clinical experience, we find it helpful to taper or stop medication with weight gaining effects38 prior to a weight‐reducing intervention, as this may enhance the weight reduction. An overview of medication that may induce weight gain is provided in Table 2.
Table 2.
Drugs that may induce weight gain
| Drug Class | Examples of Specific Agents | Estimated Weight Gaining‐Effect per Agent | References |
|---|---|---|---|
| Antidepressants | Citalopram |
26% higher chance of an episode of 5% weight gain Mean weight gain during >4 months of treatment 1.69 kg |
38, 39, 94 |
| Mirtazapine |
50% higher chance of an episode of 5% weight gain Mean weight gain 2.59 kg during >4 months treatment |
||
| Amitryptilline |
17% higher chance of an episode of 5% weight gain Mean weight gain 2.24 kg durine >4 months treatment |
||
| Paroxetine |
Mean weight gain after >4 months of treatment 2.73 kg 5% higher chance of an episode of 5% weight gain |
||
| Antipsychotics | Olanzapine |
3.8 kg to 16.2 kg in youth 29% increases ≥7% in body weight |
38, 40, 95, 96 |
| Lithium | 4‐12 kg weight increase | ||
| Clozapine | 0.9‐9.5 kg in youth | ||
| Quetiapine |
2.3‐6.1 kg in youth 25% increases ≥7% in body weight |
||
| Risperidon |
1.9‐7.2 kg in youth 18% increases ≥7% in body weight |
||
| Ziprasidone | 9.8% increases ≥7% in body weight | ||
| Anti‐epileptics | Carbamazepine | 7‐15 kg weight gain | 38, 96, 97 |
| Gabapentin | 57% gains ≥5% of baseline weight | ||
| Valproic acid |
47% gains >10% of baseline weight, 24% gains 5‐10% weight 0.5‐6 kg weight gain on average |
||
| Anti‐diabetics | Insulin | 1.78‐6 kg weight gain in the first year | 96, 98, 99 |
| SU derivates | 2‐4 kg over 1 year of treatment | ||
| Anti‐hypertensives | α‐Adrenergic blockers | 0.4‐2.0 kg | 100 |
| Β‐Adrenergic blockers | 1.2‐kg mean weight difference when compared with other antihypertensives | 44 | |
| Corticosteroids | Systemic corticosteroids |
Depending on doses, indication, and large individual variation Rheumatoid arthritis: weight increase 4% to 8% 70% of patients report weight gain |
38, 101 |
| Local corticosteroids |
Unknown association of higher BMI in women |
42 | |
| Others | Proton pump inhibitors | Possible association with weight gain | 50, 102, 103 |
| Protease inhibitors |
Lipodystrophy Average weight gain 8kg in a study of 10 patients |
38, 104, 105 | |
| Anti‐histamines | Association with higher weight and waist circumference | 38, 47, 103 |
Drugs that are frequently used in psychiatry, such as specific selective serotonin reuptake inhibitors or antipsychotic agents, are well‐known to promote weight gain. The strongest weight change is seen in amitriptyline, mirtazapine, and paroxetine.39 As for antipsychotic drugs, olanzapine and clozapine induce the highest weight gain.40 Also, several anti‐epileptic drugs should be noted. Although most prescribers are aware of these side effects, in patients with severe obesity, a medication switch to less obesogenic drugs should be considered if possible.38
It is well‐known that systemic corticosteroids can cause weight gain. For the treatment of rheumatoid arthritis, for example, it was found that low‐dose prednisone is associated with a weight increase of 4% to 8%.41 Corticosteroids can be administered in many forms. Often overlooked are the local forms, which we recently reported to be associated with increased BMI and waist circumference when compared with non‐corticosteroid users in a large population‐based cohort.42 Local corticosteroids may also have systemic effects resulting in weight gain, in a similar matter as local corticosteroids having other systemic effects, such as adrenal insufficiency,43 which needs further research. A weight gaining systemic effect of local corticosteroids is likely in patients using large quantities on a frequent basis, particularly if they show additional Cushingoid features, such as abdominal obesity, peripheral atrophy, plethora, and purple striae, as demonstrated by clinical case A.
The initiation of insulin therapy or sulphonylurea derivates in patients with type 2 diabetes can be accompanied by weight gain.38 Blood‐glucose lowering drugs without weight inducing effects, such as glucagon‐like peptide‐1 analogues, metformin, and sodium‐glucose cotransporter‐2 inhibitors,38 can be good alternatives if weight loss is desired.
Other drugs that have been shown to induce obesity are non‐selective β‐adrenergic receptor blockers.38, 44 If prescribed for hypertension, one could consider replacing them with other antihypertensive agents such as ACE‐inhibitors or angiotensin receptor blockers.44 The weight increase is more profound in metoprolol than in carvedilol. Interestingly, the weight increase was worse in subjects who already had severe obesity.45 , 46 In HIV patients, the start of protease inhibitors has also been associated with weight gain and increased deposition of visceral adipose tissue.38 Another association has been found in users of H1 antihistamines, who were more likely to be overweight than non‐users.47
Regarding hormonal contraceptives, there is no large effect on weight, although available evidence is insufficient.48 However, we cannot rule out that some women experience excessive weight gain due to individual differences.
Also, for several other agents, such as proton pump inhibitors and alpha blockers, weight gaining effects have been reported.49, 50
3.3. Genetic causes of obesity
An overview of (mono)genetic obesity disorders is summarized in Table 3. The genetics of obesity are complex. In the general population, the fat‐mass and obesity associated gene (FTO) has shown the strongest association to obesity.51 Besides these polygenic associations that have not been fully elucidated as yet, only a small percentage of the patients with obesity can be classified as having a monogenic or syndromic obesity disorder.52
Table 3.
Examples of relevant genetic obesity disorders
| Syndrome | Clinical Symptoms55 | Dysmorphic Features | Estimated Prevalence106 | Reference | |
|---|---|---|---|---|---|
| Syndromic obesity with developmental delay | Prader‐Willi syndrome |
Developmental delay and intellectual disability, (prenatal) hypotonia, feeding difficulties Failure to thrive, hyperphagia, neurologic, and cognitive disturbances Hypogonadism |
Almond‐shaped eyes, strabismus, thin upper lip, downturned corners of the mouth Short stature Genital hypoplasia |
1/15.000‐1/30.000 |
Cassidy and Driscoll107 |
| Bardet‐Biedl syndrome |
Developmental delay Intellectual disability Retinal dystrophy Renal dysfunction Hypogonadism Cardiac abnormalities |
Post‐axial polydactyly Dental crowding High‐arched palate Hypodontia Malocclusion Enamel hypoplasia Micropenis |
1/160.000 (northern European) 1/13.500 (specific populations) |
Forsythe et al108 | |
| 16p11.2 deletion syndrome |
Developmental delay Mild intellectual disability Autism spectrum disorders |
Variable presentation Macrocephaly |
3 in 10.000 | Dell'edera et al109 | |
| Albright hereditary osteodystrophy |
Short stature, subcutaneous ossifications Maternally inherited GNAS mutations: Hormone resistance (eg, parathyroid hormone)sometimes developmental delay and intellectual disability |
Round facies Brachydactyly fourth/fifth metacarpals | 0.79 in 100.000 | Haldeman‐Englert et al58 | |
|
Genetic obesity without developmental delay |
MC4R deficiency |
Hyperphagia Accelerated linear growth Disproportionate hyperinsulinemia Low/normal blood pressure |
Not apparent | 2%‐5% of subjects with extreme pediatric obesity | Martinelli et al110, 111 |
| Leptin (receptor) deficiency |
Extreme hyperphagia Frequent infections Hypogonadotropic hypogonadism Mild hypothyroidism |
Not apparent |
Leptin receptor deficiency: 2%‐3% in severely obese subjects Leptin deficiency: Fewer than 100 patients worldwide |
Farooqi et al112 | |
| POMC deficiency | Hyperphagia, ACTH deficiency, pale skin, and red hair | Not apparent | Unknown | Krude et al113 | |
| PCSK1 deficiency |
Adrenal, gonadotropic, somatotropic, and thyrotropic insufficiency severe malabsorptive neonatal diarrhea central diabetes insipidus |
Not apparent | Unknown | RamosMolina et al114 |
Screening for these conditions is not routinely done in clinical practice. As new therapies are arising that target specific types of obesity, we here plead for screening for genetic obesity in a subgroup of patients who have a high clinical suspicion for these types of genetic obesity. A recent study found a confirmed diagnosis of genetic obesity in 3.9% of patients who were clinically suspected of genetic obesity.53 Indications for genetic screening include an early age of onset, below 5 years of age,54, 55 (or in adult populations a prepubertal onset), a family history with striking weight differences between family members (which may indicate monogenic obesity), and severe hyperphagia (which can be seen in monogenetic obesity‐with and without intellectual deficit). Furthermore, characteristics such as intellectual deficit or developmental delay, congenital malformations, visual impairment and/or deafness, and abnormal growth parameters (head circumference and height) may be indicative for syndromic obesity. Examples of syndromic obesity are Prader‐Willi syndrome (characterized by hypotonia and feeding problems in infancy, and later in life hyperphagia and obesity, short stature, intellectual deficit and hypogonadotropic hypogonadism56), Bardet‐Biedl syndrome (intellectual deficit, retinal dystrophy or pigmentary retinopathy, polydactyly, hypogonadism, and nephropathy), and the 16p11.2 deletion syndrome (intellectual deficit, speech development problems, autism, macrocephaly, and epilepsy). There is a large variability of these symptoms among affected patients.57 Mild to moderate intellectual disability is also seen in patients with pseudohypoparathyroidism type 1 (PHP1a), caused by maternally inherited heterozygous mutations in GNAS. PHP1a is associated with the clinical phenotype of Albright's hereditary osteodystrophy (AHO), which encompasses short stature, round facies, and skeletal abnormalities58
Monogenic (non‐syndromic) causes of obesity are characterized by a young age of onset and hyperphagia,12, 54 with usually no intellectual deficit. Additionally, other clinical signs of monogenetic obesity may differ depending on the affected gene. These include red or ruddy hair, pale skin, and adrenocorticotropic hormone (ACTH) deficiency (pro‐opiomelanocortin [POMC] gene defects), central hypothyroidism, hypogonadotropic hypogonadism and frequent infections (leptin deficiency, caused by autosomal recessive LEP gene mutations), increased linear growth and increased lean mass, severe hyperinsulinemia, and mild central hypothyroidism (caused by autosomal dominant or recessive MC4R mutations) and neonatal diarrhea, recurrent hypoglycemia, and global endocrine dysfunction (caused by prohormone convertase‐1 [PCSK1] mutations).54, 59
3.4. Neuroendocrine causes
3.4.1. Hormonal causes
A relatively sudden increase in weight may suggest a neuroendocrine cause. We screen for hypothyroidism, as this is associated with a modest weight gain. This is especially recommended if patients present with other symptoms such as dry skin, feeling cold, etc. However, the weight gain in hypothyroidism seems mostly due to additional edema.60 Also, obesity is often associated with a slightly increased TSH that is most often the result of excess adipose tissue rather than the cause of obesity. This can be explained by the presence of peripheral thyroid resistance and also by increased levels of leptin, stimulating TRH and subsequently TSH.9, 10 Weight loss usually reverses this form of hyperthyrotropinemia.61
To identify Cushing syndrome (CS), specific signs of CS including easy bruising, facial plethora, proximal myopathy, and recent purple striae62 should be considered, as most patients with obesity will have the more non‐specific CS signs such as central obesity, fatigue, hypertension, and decreased libido. Due to the large number of corticosteroid users,63, 64 iatrogenic CS should also be considered.
Another common endocrine condition associated with obesity is the polycystic ovarian syndrome (PCOS), a constellation of hyperandrogenism, oligoovulation or anovulation, and polycystic ovaries. Male hypogonadism has a complex, bidirectional relation with obesity. Testosterone therapy in patients with obesity or diabetes with evident testosterone deficiency causes improvement of body composition and components of the metabolic syndrome.8, 65 On the other hand, obesity induces hypogonadotropic hypogonadism, and a healthy diet and physical activity can increase bound and unbound testosterone levels and completely normalize the hypogonadism.66
Women may report weight gain after pregnancy67 or menopause.68 The average weight change from preconception to the first year postpartum is referred to as “postpartum weight retention.” This is on average relatively small (0.5 to 1.5 kg). However, there is a large variability, as 13% to 20% of women are 5 kg or more above their preconception weight by 1‐year postpartum.69 Although menopause is frequently reported as a contributing factor by women, and experimental evidence suggests estrogen depletion is associated with abdominal fat accumulation, epidemiological evidence in humans indicates that the steady weight gain of 0.5 kg annually is due to age rather than the menopause itself.70
In specific patients with a history of pituitary disease, surgery or irradiation in these areas, severe head trauma, or evidence of other pituitary hormone deficiencies,71 endocrine evaluation is indicated, including growth hormone (GH) deficiency as it may contribute to obesity and treatment options are available.72, 73
3.4.2. Hypothalamic disorders
Hypothalamic obesity, typically accompanied by hyperphagia, can occur after various insults leading to damage of the hypothalamic region. It is seen in patients with abnormalities in the hypothalamic region, eg, craniopharyngeoma (especially following surgery), inflammatory processes such as sarcoidosis and tuberculosis, vascular damage, head trauma, or after cranial radiotherapy, but also some of the genetic mutations that were previously mentioned can be considered hypothalamic obesity.74 In severe cases, it can lead to multiple endocrine symptoms such as impaired reproductive function with amenorrhea or impotence, diabetes insipidus, and thyroid or adrenal insufficiency (obesity that results in hormonal abnormalities is discussed in the previous paragraph). Also, neurological symptoms can be seen, including convulsions, hypothermia or hyperthermia, or somnolence.75
3.5. Mental factors
In patients with obesity, attention to mental factors should be paid. Numerous studies showed that depression is linked to obesity, in a bidirectional manner.76, 77, 78 This is not surprising, as changes in food intake are considered symptoms of depression.79 The associations are stronger for patients with a symptom profile that is often labeled as “atypical.”78 Also, anxiety disorders are cross‐sectionally associated with obesity.80
Binge‐eating disorder81 is characterized by recurrent binge‐eating episodes where more food is consumed than is normal for most people and where feelings of lack of control and distress play a role. Importantly, binge‐eating can also be a sign of hyperphagia and may thus be a diagnostic clue for either genetic or hypothalamic obesity, as demonstrated in Clinical Case B.
Evidence is mounting that stress leads to more appetite (in comfort food), induces abdominal obesity,24 and may counteract the effects of a healthy diet.82, 83, 84 Additionally, the weight stigma that individuals with obesity often suffer from may also lead to extra weight gain.85 It is therefore conceivable that a non‐stigmatizing attitude, as well as stress reduction, is beneficial when treating obesity.
Furthermore, events during early life have been identified as risk factors for obesity. Adolescents with a history of childhood sexual abuse have a higher risk of obesity (in men) or disordered eating (in women)86
4. LABORATORY DIAGNOSTICS AND FURTHER EVALUATION
General laboratory evaluation can be considered in all patients with obesity, including fasting glucose (and insulin in case of acanthosis nigricans), lipids, liver enzymes, and thyroid screening (TSH, and FT4 on indication).5 In case of clinical clues, this can be extended by testing gonadal function and/or Cushing diagnostics. Diagnostics for (mono)genetic obesity should be reserved for individuals with a high clinical suspicion (eg, early onset obesity, with or without intellectual deficit, dysmorphic features/congenital malformations, behavioral problems, hyperphagia, and/or striking family history, Figure 1, 53).
5. SOCIETY‐RELATED OBESOGENIC FACTORS AND FUTURE TARGETS FOR PUBLIC HEALTH INTERVENTIONS
The assessment of obesity can be complicated further by considering disruptors of energy homeostasis on a societal level. These currently have limited value in clinical practice but are interesting possible targets for public health interventions. These include endocrine disruptors, such as chemicals in pesticides/herbicides, industrial and household products, plastics, detergents, flame retardants, and ingredients in personal care products, that can affect the endocrine system and interfere with various metabolic processes.87, 88 In addition, both low birthweight and high birthweight in boys and high birth weight in girls have been associated to obesity,89 indicating that intra‐uterine factors may be important to weight. Also, early childhood infections have recently been shown to contribute to obesity.90 In this context, an interesting emerging field of research is “infectobesity,” which is the study of microbial agents as a cause of obesity. For example, intriguing associations between obesity and the adenovirus‐36 have been found.91 The changing gut microbiome seems more and more important in the pathogenesis of obesity.92
6. CONCLUSIONS
Obesity is a complex clinical problem where adequate diagnostics are often overlooked. We plead for a comprehensive approach of obesity, including adequate diagnostics prior to treatment. This includes, besides evaluation of lifestyle factors, a detailed past and present medical history, medication use, a global outline of the sociocultural environment, and a thorough physical examination. In case of abnormalities suggestive of an underlying disease, we recommend specific testing for these causes, as some conditions may be treatable. Furthermore, modifiable contributing factors, such as the use of weight gaining medication, lack of sleep, chronic stress etc. may be optimized before starting a lifestyle intervention or bariatric surgery. This may lead to more effective treatment for individuals with obesity. Additionally, knowing the cause of the obesity can help to reduce the social stigma that many individuals with obesity suffer from.
ACKNOWLEDGEMENTS
EFCvR is supported by a Vidi grant from the Netherlands Organization of Scientific Research NWO (grant number: 91716453). ELTvdA and EFCvR are funded by the Elisabeth Foundation.
We thank the patients whose history was written above and who gave permission to publish this article.
van der Valk ES, van den Akker ELT, Savas M, et al. A comprehensive diagnostic approach to detect underlying causes of obesity in adults. Obesity Reviews. 2019;20:795–804. 10.1111/obr.12836
REFERENCES
- 1. Bray GA, Kim KK, Wilding JPH, World OF. Obesity: a chronic relapsing progressive disease process. A position statement of the World obesity federation. Obes Rev. 2017;18(7):715‐723. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organisation . Obesity and Overweight fact sheet. june 2016 2016.
- 3. Collaborators GBDO . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13‐e115. [DOI] [PubMed] [Google Scholar]
- 5. Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of endocrinology comprehensive clinical practice guidelines for medical Care of Patients with obesity. Endocr Pract. 2016;22(Suppl 3):1‐203. [DOI] [PubMed] [Google Scholar]
- 7. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. Circulation. 2014;129(25 Suppl 2):S102‐S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saad F, Yassin A, Doros G, Haider A. Effects of long‐term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I‐III: observational data from two registry studies. Int J Obes (Lond). 2016;40(1):162‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab. 2010;95(8):3614‐3617. [DOI] [PubMed] [Google Scholar]
- 10. Sanyal D, Raychaudhuri M. Hypothyroidism and obesity: an intriguing link. Indian J Endocrinol Metab. 2016;20(4):554‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hainerova IA, Lebl J. Treatment options for children with monogenic forms of obesity. World Rev Nutr Diet. 2013;106:105‐112. [DOI] [PubMed] [Google Scholar]
- 12. Alsters S. Genetic Analysis of Extreme Obesity. London: Department of Medicine, Imperial College London; 2016. [Google Scholar]
- 13. Fani L, Bak S, Delhanty P, van Rossum EF, van den Akker EL. The melanocortin‐4 receptor as target for obesity treatment: a systematic review of emerging pharmacological therapeutic options. Int J Obes (Lond). 2014;38(2):163‐169. [DOI] [PubMed] [Google Scholar]
- 14. Kühnen P, Clément K, Wiegand S, et al. Proopiomelanocortin deficiency treated with a melanocortin‐4 receptor agonist. N Engl J Med. 2016;375(3):240‐246. [DOI] [PubMed] [Google Scholar]
- 15. Sharma AM, Padwal R. Obesity is a sign―over‐eating is a symptom: an aetiological framework for the assessment and management of obesity. Obes Rev. 2010;11(5):362‐370. [DOI] [PubMed] [Google Scholar]
- 16. Romieu I, Dossus L, Barquera S, et al. Energy balance and obesity: what are the main drivers? Cancer Causes Control. 2017;28(3):247‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heymsfield SB, Harp JB, Reitman ML, et al. Why do obese patients not lose more weight when treated with low‐calorie diets? A mechanistic perspective. Am J Clin Nutr. 2007;85(2):346‐354. [DOI] [PubMed] [Google Scholar]
- 18. Macht M. How emotions affect eating: a five‐way model. Appetite. 2008;50(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 19. Cappuccio FP, Taggart FM, Kandala NB, et al. Meta‐analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring). 2008;16(3):643‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bayon V, Leger D, Gomez‐Merino D, Vecchierini MF, Chennaoui M. Sleep debt and obesity. Ann Med. 2014;46(5):264‐272. [DOI] [PubMed] [Google Scholar]
- 22. Minkel J, Moreta M, Muto J, et al. Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Psychol. 2014;33(11):1430‐1434. [DOI] [PubMed] [Google Scholar]
- 23. Manenschijn L, Van Kruysbergen RG, De Jong FH, Koper JW, Lamberts SWJ, Van Rossum EFC. Shift work at young age is associated with elevated long‐term cortisol levels and body mass index. Endocr Rev. 2012;33(3):E1862‐E1865. [DOI] [PubMed] [Google Scholar]
- 24. van der Valk ES, Savas M, van Rossum EFC. Stress and obesity: are there more susceptible individuals? Curr Obes Rep. 2018;7(2):193‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Broussard JL, Van Cauter E. Disturbances of sleep and circadian rhythms: novel risk factors for obesity. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):353‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McHill AW, Wright KP Jr. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes Rev. 2017;18(Suppl 1):15‐24. [DOI] [PubMed] [Google Scholar]
- 27. St‐Onge MP, Shechter A. Sleep disturbances, body fat distribution, food intake and/or energy expenditure: pathophysiological aspects. Horm Mol Biol Clin Invest. 2014;17(1):29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shechter A. Effects of continuous positive airway pressure on energy balance regulation: a systematic review. Eur Respir J. 2016;48(6):1640‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shechter A. Obstructive sleep apnea and energy balance regulation: a systematic review. Sleep Med Rev. 2017;34:59‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drager LF, Brunoni AR, Jenner R, Lorenzi‐Filho G, Bensenor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta‐analysis of randomised trials. Thorax. 2015;70(3):258‐264. [DOI] [PubMed] [Google Scholar]
- 31. Nymo S, Coutinho SR, Torgersen LH, et al. Timeline of changes in adaptive physiological responses, at the level of energy expenditure, with progressive weight loss. Br J Nutr. 2018;120(2):141‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Myers A, Gibbons C, Finlayson G, Blundell J. Associations among sedentary and active behaviours, body fat and appetite dysregulation: investigating the myth of physical inactivity and obesity. Br J Sports Med. 2017;51(21):1540‐1544. [DOI] [PubMed] [Google Scholar]
- 33. Villablanca PA, Alegria JR, Mookadam F, Holmes DR Jr, Wright RS, Levine JA. Nonexercise activity thermogenesis in obesity management. Mayo Clin Proc. 2015;90(4):509‐519. [DOI] [PubMed] [Google Scholar]
- 34. Kim SH, Plutzky J. Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab J. 2016;40(1):12‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoneshiro T, Aita S, Matsushita M, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123(8):3404‐3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harris KK, Zopey M, Friedman TC. Metabolic effects of smoking cessation. Nat Rev Endocrinol. 2016;12(5):299‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bush T, Lovejoy JC, Deprey M, Carpenter KM. The effect of tobacco cessation on weight gain, obesity, and diabetes risk. Obesity (Silver Spring). 2016;24(9):1834‐1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342‐362. [DOI] [PubMed] [Google Scholar]
- 39. Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta‐analysis. J Clin Psychiatry. 2010;71(10):1259‐1272. [DOI] [PubMed] [Google Scholar]
- 40. Allison DB, Casey DE. Antipsychotic‐induced weight gain: a review of the literature. J Clin Psychiatry. 2001;62(Suppl 7):22‐31. [PubMed] [Google Scholar]
- 41. Da Silva JAP, Jacobs JWG, Kirwan JR, et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data. Ann Rheum Dis. 2006;65(3):285‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Savas M, Muka T, Wester VL, et al. Associations between systemic and local corticosteroid use with metabolic syndrome and body mass index. J Clin Endocrinol Metab. 2017;102(10):3765‐3774. [DOI] [PubMed] [Google Scholar]
- 43. Broersen LH, Pereira AM, Jorgensen JO, Dekkers OM. Adrenal insufficiency in corticosteroids use: systematic review and meta‐analysis. J Clin Endocrinol Metab. 2015;100(6):2171‐2180. [DOI] [PubMed] [Google Scholar]
- 44. Sharma AM, Pischon T, Hardt S, Kunz I, Luft FC. Hypothesis: beta‐adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37(2):250‐254. [DOI] [PubMed] [Google Scholar]
- 45. Messerli FH, Bell DS, Fonseca V, et al. Body weight changes with beta‐blocker use: results from GEMINI. Am J Med. 2007;120(7):610‐615. [DOI] [PubMed] [Google Scholar]
- 46. Manrique C, Whaley‐Connell A, Sowers JR. Nebivolol in obese and non‐obese hypertensive patients. J Clin Hypertens (Greenwich). 2009;11(6):309‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ratliff JC, Barber JA, Palmese LB, Reutenauer EL, Tek C. Association of prescription H1 antihistamine use with obesity: results from the National Health and nutrition examination survey. Obesity (Silver Spring). 2010;18(12):2398‐2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gallo MF, Lopez LM, Grimes DA, Carayon F, Schulz KF, Helmerhorst FM. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014;1 CD003987. 10.1002/14651858.CD003987.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cheskin LJ, Bartlett SJ, Zayas R, Twilley CH, Allison DB, Contoreggi C. Prescription medications: a modifiable contributor to obesity. South Med J. 1999;92(9):898‐904. [DOI] [PubMed] [Google Scholar]
- 50. Yoshikawa I, Nagato M, Yamasaki M, Kume K, Otsuki M. Long‐term treatment with proton pump inhibitor is associated with undesired weight gain. World J Gastroenterol. 2009;15(38):4794‐4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ehrlich AC, Friedenberg FK. Genetic associations of obesity: the fat‐mass and obesity‐associated (FTO) gene. Clin Transl Gastroenterol. 2016;7(1):e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bays H, Scinta W. Adiposopathy and epigenetics: an introduction to obesity as a transgenerational disease. Curr Med Res Opin. 2015;31(11):2059‐2069. [DOI] [PubMed] [Google Scholar]
- 53. Kleinendorst L, Massink MPG, Cooiman MI, et al. Genetic obesity: next‐generation sequencing results of 1230 patients with obesity. J Med Genet. 2018;55(9):578‐586. [DOI] [PubMed] [Google Scholar]
- 54. Farooqi IS. The severely obese patient—a genetic work‐up. Nat Clin Pract Endocrinol Metab. 2006;2(3):172‐177. [DOI] [PubMed] [Google Scholar]
- 55. Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity—assessment, treatment, and prevention: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102(6):2123‐2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Holm VA, Cassidy SB, Butler MG, et al. Prader‐Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91(2):398‐402. [PMC free article] [PubMed] [Google Scholar]
- 57. Miller DT, Chung W, Nasir R. 16p11.2 Recurrent Microdeletion In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReviews®. WA: University of Washington, Seattle; 2015. [Updated 2015 Dec 10] ed. [Google Scholar]
- 58. Haldeman‐Englert CR, Hurst ACE, Levine MA. Disorders of GNAS Inactivation. 1993.
- 59. Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085‐1095. [DOI] [PubMed] [Google Scholar]
- 60. Laurberg P, Knudsen N, Andersen S, Carle A, Pedersen IB, Karmisholt J. Thyroid function and obesity. Eur Thyroid J. 2012;1(3):159‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reinehr T, de Sousa G, Andler W. Hyperthyrotropinemia in obese children is reversible after weight loss and is not related to lipids. J Clin Endocrinol Metab. 2006;91(8):3088‐3091. [DOI] [PubMed] [Google Scholar]
- 62. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing's syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Savas M, Wester VL, Staufenbiel SM, et al. Systematic evaluation of corticosteroid use in obese and non‐obese individuals: a multi‐cohort study. Int J Med Sci. 2017;14(7):615‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Savas MMT, Wester VL, van den Akker ELT, et al. Associations between systemic and local corticosteroid use with metabolic syndrome and body mass index. J Clin Endocrinol Metabol. 2017;102(10):3765‐3774. [DOI] [PubMed] [Google Scholar]
- 65. Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154(6):899‐906. [DOI] [PubMed] [Google Scholar]
- 66. Corona G, Rastrelli G, Monami M, et al. Body weight loss reverts obesity‐associated hypogonadotropic hypogonadism: a systematic review and meta‐analysis. Eur J Endocrinol. 2013;168(6):829‐843. [DOI] [PubMed] [Google Scholar]
- 67. Gunderson EP, Sternfeld B, Wellons MF, et al. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring). 2008;16(5):1078‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92(3):895‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009;36(2):317‐332. ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Davis SR, Castelo‐Branco C, Chedraui P, et al. Understanding weight gain at menopause. Climacteric. 2012;15(5):419‐429. [DOI] [PubMed] [Google Scholar]
- 71. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Endocrine S. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(6):1587‐1609. [DOI] [PubMed] [Google Scholar]
- 72. Maison P, Griffin S, Nicoue‐Beglah M, et al. Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH‐deficient adults: a metaanalysis of blinded, randomized, placebo‐controlled trials. J Clin Endocrinol Metab. 2004;89(5):2192‐2199. [DOI] [PubMed] [Google Scholar]
- 73. Cenci MC, Conceicao FL, Soares DV, et al. Impact of 5 years of growth hormone replacement therapy on cardiovascular risk factors in growth hormone‐deficient adults. Metabolism. 2008;57(1):121‐129. [DOI] [PubMed] [Google Scholar]
- 74. Bereket A, Kiess W, Lustig RH, et al. Hypothalamic obesity in children. Obes Rev. 2012;13(9):780‐798. [DOI] [PubMed] [Google Scholar]
- 75. Lee M, Korner J. Review of physiology, clinical manifestations, and management of hypothalamic obesity in humans. Pituitary. 2009;12(2):87‐95. [DOI] [PubMed] [Google Scholar]
- 76. Faith MS, Butryn M, Wadden TA, Fabricatore A, Nguyen AM, Heymsfield SB. Evidence for prospective associations among depression and obesity in population‐based studies. Obes Rev. 2011;12(5):e438‐e453. [DOI] [PubMed] [Google Scholar]
- 77. de Wit L, Luppino F, van Straten A, Penninx B, Zitman F, Cuijpers P. Depression and obesity: a meta‐analysis of community‐based studies. Psychiatry Res. 2010;178(2):230‐235. [DOI] [PubMed] [Google Scholar]
- 78. Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2018;24:18‐38. [DOI] [PubMed] [Google Scholar]
- 79. Association AP . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- 80. Rajan TM, Menon V. Psychiatric disorders and obesity: a review of association studies. J Postgrad Med. 2017;63(3):182‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dallman MF. Stress‐induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21(3):159‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tamashiro KL, Sakai RR, Shively CA, Karatsoreos IN, Reagan LP. Chronic stress, metabolism, and metabolic syndrome. Stress. 2011;14(5):468‐474. [DOI] [PubMed] [Google Scholar]
- 84. Kiecolt‐Glaser JK, Fagundes CP, Andridge R, et al. Depression, daily stressors and inflammatory responses to high‐fat meals: when stress overrides healthier food choices. Mol Psychiatry. 2017;22(3):476‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tomiyama AJ. Weight stigma is stressful. A review of evidence for the cyclic obesity/weight‐based stigma model. Appetite. 2014;82:8‐15. [DOI] [PubMed] [Google Scholar]
- 86. Fuemmeler BF, Dedert E, McClernon FJ, Beckham JC. Adverse childhood events are associated with obesity and disordered eating: results from a U.S. population‐based survey of young adults. J Trauma Stress. 2009;22(4):329‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bergman Å, Heindel JJ, Jobling S, Kidd KA, Zoeller RT, Programme WHoatUNE . Endocrine Disrupting Chemicals. 2012.
- 88. Darbre PD. Endocrine disruptors and obesity. Curr Obes Rep. 2017;6(1):18‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Qiao Y, Ma J, Wang Y, et al. Birth weight and childhood obesity: a 12‐country study. Int J Obes Suppl. 2015;5(Suppl 2):S74‐S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li DK, Chen H, Ferber J, Odouli R. Infection and antibiotic use in infancy and risk of childhood obesity: a longitudinal birth cohort study. Lancet Diabetes Endocrinol. 2017;5(1):18‐25. [DOI] [PubMed] [Google Scholar]
- 91. Xu MY, Cao B, Wang DF, et al. Human adenovirus 36 infection increased the risk of obesity: a meta‐analysis update. Medicine (Baltimore). 2015;94(51):e2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the gut microbiome in the pathogenesis of obesity and obesity‐related metabolic dysfunction. Gastroenterology. 2017;152(7):1671‐1678. [DOI] [PubMed] [Google Scholar]
- 93. Wu FC, Tajar A, Beynon JM, et al. Identification of late‐onset hypogonadism in middle‐aged and elderly men. N Engl J Med. 2010;363(2):123‐135. [DOI] [PubMed] [Google Scholar]
- 94. Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years' follow‐up: population based cohort study. BMJ. 2018;361:k1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Maayan L, Correll CU. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol. 2011;21(6):517‐535. [DOI] [PubMed] [Google Scholar]
- 96. Medici V, McClave SA, Miller KR. Common medications which Lead to unintended alterations in weight gain or organ Lipotoxicity. Curr Gastroenterol Rep. 2016;18(1):2. [DOI] [PubMed] [Google Scholar]
- 97. Lampl Y, Eshel Y, Rapaport A, Sarova‐Pinhas I. Weight gain, increased appetite, and excessive food intake induced by carbamazepine. Clin Neuropharmacol. 1991;14(3):251‐255. [DOI] [PubMed] [Google Scholar]
- 98. Hodish I. Insulin therapy, weight gain and prognosis. Diabetes Obes Metab. 2018;20(9):2085‐2092. [DOI] [PubMed] [Google Scholar]
- 99. Hemmingsen B, Schroll JB, Wetterslev J, et al. Sulfonylurea versus metformin monotherapy in patients with type 2 diabetes: a Cochrane systematic review and meta‐analysis of randomized clinical trials and trial sequential analysis. CMAJ Open. 2014;2(3):E162‐E175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bryson CM, Psaty BM. A review of the adverse effects of peripheral Alpha‐1 antagonists in hypertension therapy. Curr Control Trials Cardiovasc Med. 2002;3(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Curtis JR, Westfall AO, Allison J, et al. Population‐based assessment of adverse events associated with long‐term glucocorticoid use. Arthritis Rheum. 2006;55(3):420‐426. [DOI] [PubMed] [Google Scholar]
- 102. Czwornog JL, Austin GL. Association of Proton Pump Inhibitor (PPI) use with energy intake, physical activity, and weight gain. Nutrients. 2015;7(10):8592‐8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ward EK, Jensen‐Otsu E, Schoen JA, Rothchild K, Mitchell B, Austin GL. Acid suppression medications are associated with suboptimal weight loss after laparoscopic roux‐en‐Y gastric bypass in patients older than 40 years. Surg Obes Relat Dis. 2015;11(3):585‐590. [DOI] [PubMed] [Google Scholar]
- 104. Anuurad E, Bremer A, Berglund L. HIV protease inhibitors and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17(5):478‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Willig AL, Overton ET. Metabolic complications and glucose metabolism in HIV infection: a review of the evidence. Curr HIV/AIDS Rep. 2016;13(5):289‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Huvenne H, Dubern B, Clement K, Poitou C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obes Facts. 2016;9(3):158‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cassidy SB, Driscoll DJ. Prader‐Willi syndrome. Eur J Hum Genet. 2009;17(1):3‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Forsythe E, Beales PL. Bardet‐Biedl syndrome. Eur J Hum Genet. 2013;21(1):8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dell'Edera D, Dilucca C, Allegretti A, et al. 16p11.2 microdeletion syndrome: a case report. J Med Case Reports. 2018;12(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Martinelli CE, Keogh JM, Greenfield JR, et al. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J Clin Endocrinol Metab. 2011;96(1):E181‐E188. [DOI] [PubMed] [Google Scholar]
- 111. Vollbach H, Brandt S, Lahr G, et al. Prevalence and phenotypic characterization of MC4R variants in a large pediatric cohort. Int J Obes (Lond). 2017;41(1):13‐22. [DOI] [PubMed] [Google Scholar]
- 112. Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356(3):237‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Krude H, Biebermann H, Gruters A. Mutations in the human proopiomelanocortin gene review. Ann N Y Acad Sci. 2003;994:233‐239. [DOI] [PubMed] [Google Scholar]
- 114. Ramos‐Molina B, Martin MG, Lindberg I. PCSK1 variants and human obesity. Prog Mol Biol Transl Sci. 2016;140:47‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]


