Abstract
Introduction
Visual impairment (VI) is one of the major public health problems in the world. It is highly prevalent among children in sub-Saharan countries, including Ethiopia. Worldwide, the magnitude of VI among school-age children is 1%–10%. However, there was limited information regarding the prevalence and associated factors of VI among school-age children in the study area, which is essential to plan and implement appropriate interventions.
Objective
The aim of this study was to determine the prevalence and associated factors of VI among school-age children livin g in Bahir Dar city, northwest Ethiopia.
Methods
A community-based cross-sectional study was done on a sample of 632 school-age children selected by multistage sampling in Bahir Dar from April 30 to May 15, 2018. Data were collected through interviews and physical examinations. Face-to-face interviews were done with a pretested semistructured questionnaire. Physical examinations were done with visual acuity measures and assessment of ocular pathology by optometrists. Data were entered into Epi Info 7 and exported to and analyzed with SPSS 20. Binary logistic regression was fitted, and variables with P<0.05 in the multivariate model were considered statistically significant.
Results
A total of 601 study subjects were included in this study, giving a response rate of 95.2%. The median age was 13 (IQR 11–16) years, and 303 (50.3%) were male. Prevalence of VI was 52 (8.7%, 95% CI 6.2%–10.7%). In multivariate analysis, prematurity [AOR 2.8 (95% CI 1.19–6.83)], admission to a neonatal intensive-care unit (AOR 5.5, 95% CI 2.01–15.15), having a parent with VI (AOR 1.8, 95% CI 0.13–0.97), watching television from <2 m (AOR 8.7, 95% CI 1.49–18.24), and mobile-phone exposure >4 hours per day (AOR 1.6, 95% CI 1.32–4.45) were factors significantly associated with VI.
Conclusion
The prevalence of VI among school-age children in Bahir Dar was significant. Premature birth, admission to a neonatal intensive-care unit, having a parent with VI, watching television from <2 m, and mobile exposure >4 hours per day were significantly associated.
Keywords: school-age children, northwest Ethiopia, visual impairment
Introduction
Visual impairment (VI) is a significant loss of vision, clinically defined as presenting distance visual acuity <6/18 in the better eye. It can be classified as mild, moderate, severe, and blindness 1–5.1,2 Of 285 million visually impaired people worldwide,3 18 million are younger than 15 year and a child goes blind every minute.4 The prevalence of VI is higher in developing countries and has been estimated to be 5.3% in Ethiopia.5,6 Worldwide, the magnitude of VI among school-age children is 1%–10%.2,7−15 In reality, 75%–90% of all learning activity is done with vision in the classroom, and thus VI drastically affects the academic performance and social activity of children.7 The effects of VI in children further extend to the well-being of individuals and families and social welfare. Loss of productivity and payment for treatment and visual aids has human and socioeconomic consequences, which in turn perpetuate ill health, leading to death.10 If early diagnosis and treatment are made, the majority of VI can be corrected easily.11,12 Low birth weight,13 white ethnicity, premature birth, and admission to a neonatal intensive-care unit (NICU),14 large head circumstance, presence of congenital abnormalities and cesarean-section delivery,15–17 maternal alcohol consumption, families with lower parental education, and positive family history of VI,18,19 being female, age range of 10–13 years, learning in public schools, watching television from <2 m,8,9 duration of television exposure, distance of television exposure, mobile-phone exposure, medical visit history,2,5,7 and lower family monthly income20 have been found to be positively associated with VI.
Although VI and its associated factors have well studied in developed countries, there are limited data for Ethiopia, and none for northwest Ethiopia. Identifying risk factors and estimating the prevalence of VI among school-age children are important for the establishment and implementation of prevention strategies and programs. More action needs to be undertaken in preventing VI, because ocular problems may cause permanent blindness and child mortality.8,15–17,21 The prevalence of VI among school-age children in Ethiopia is not well established, and there have been limited surveys done at the community level o identify factors associated with VI in school-age children. This study determines the prevalence of VI and identifies factors associated with VI among school-age children in Bahir Dar city, which could be used as baseline data to facilitate health-care planning and implementation.
Methods
A community based cross sectional study was conducted in Bahir Dar city, northwest Ethiopia from April 30 to May 15, 2018. Bahir Dar is located 578 km from the capital city, Addis Ababa, with a population of about 243,300.22 It has six subcities and 17 kebele, hosting approximately 53,725 households and 100,984 children <19 years old.23 There are three governmental hospitals, three private general hospitals, and two private eye clinics that provide eye-care services.22
All children aged 6–18 years old who had been living in Bahir Dar > 6 months, except those with recent ocular trauma or surgery, were included in the study.
Sample size was determined with a single population-proportion formula: 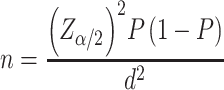
where Z had a 95% CI of 1.96, P the proportion of VI (7.24%) from a similar study in Adama, Ethiopia of school-age children (0.0724),9 and d maximum tolerable error (marginal error) of 3% = 0.03). By adding a 10% nonresponse rate and using a design effect of 2, the final sample size was 632. Multistage sampling using two sampling stages process was used. First, four of 17 kebele were selected using simple random sampling from a census list obtained from the Bahir Dar City Statistical Agency. In four selected kebele, there was a total population of 44,438 and total households of 12,015.23 Then, systematic random sampling was used to select participant households proportionally with a sampling fraction of 19 to get one child. If there were more than one child in the selected house, one child was selected randomly, and if the selected child was not present, the selected house was revisited. If the selected house had no school-age children, the next household was visited for school-age children.
Presenting distance visual acuity was defined as distance visual acuity without any correction in each eye. VI was considered when presenting distance visual acuity in the better eye was <6/18.25,26 This was categorized in to three categories: moderate VI — presenting distance VA <6/18 and ≥6/60 in the better eye (category 1);27severe VI — presenting distance VA <6/60 and ≥3/60 in the better eye (category 2);25 and blindness — presenting distance VA <3/60 in the better eye (category 3).26Positive family ocular history was defined as history of ocular problems in any first-degree relative.28 School-age children were defined as age 6–18 years.29Drinking alcohol was categorized as nondrinkers (people who explicitly recorded zero for current consumption of any alcoholic beverage and zero or blank for previous drinking) and drinkers (those who reported drinking any alcoholic beverage at least three times per week and above).30
An English version of the questionnaire was prepared and translated into Amharic (local language). The Amharic version of the questionnaire, Snellen charts, and torchlight were used to collect data. Interviews were conducted with children aged 12 years and above, while for those <12 years of age, the parent or guardian was contacted. Five trained MSc optometrists participated in data collection. Pretesting was conducted in 5% of the sample in an area with a similar setting outside the study area (Gondar city). Training was given to data collectors and supervisors. There was close supervision by supervisors. The collected data were checked for completeness, accuracy, and clarity by the principal investigator and supervisors on a daily basis. Data cleanup and cross-checking was done before analysis.
Collected data were entered into Epi Info 7 and exported to and analyzed with SPSS 20. Summary statistics, frequencies, and cross-tabulations were performed, Bivariate and multivariate logistic regression models were employed to identify associations between independent variables and a dichotomous outcome variable. Variables with p<20% in the bivariate analysis were taken for further multivariate analysis with backward logistic regression. The Hosmer–Lemeshow model was used to check model fitness. Multicollinearity between independent variables were checked by variance inflation factor. AORs with 95% CIs were used to identify significant predictors of the outcome variable. P<0.05 was considered statistically significant
Before the study, ethical clearance was obtained from the University of Gondar College of Medicine and Health Sciences and comprehensive specialized hospital and School Of Medicine Ethical Review Committee, and a support letter was obtained from Bahir Dar kebele administrators. Verbal assent was obtained from all study participants and consent was obtained from parents/guardians after a detailed explanation of the study. The University of Gondar College of Medicine and Health Sciences and comprehensive specialized hospital and School Of Medicine Ethical Review Committee had approved the taking of verbal consent from parents and guardians. Those children with VI were managed at Felege Hiwot Referral Hospital after creation of a referral system by communication with the hospital administrators.
Results
Sociodemographic Characteristics Of The Study Participants
A total of 601 study subjects were included for a response rate of 95.1%. The mean age of participants was 13.14±2.82 year. Half the study participants (50.3%) were males. The majority (84.9%) of study participants were Orthodox Christians and Amhara (93.8%) in ethnicity. About three-quarters of children (75%) and less than a third of household heads (29%) had finished primary school (Table 1).
Table 1.
Sociodemographic Characteristics Of Study Participants (n=601)
| Frequency | Percentage | |
|---|---|---|
| Age, years | ||
| 6–9 | 45 | 7.5 |
| 10–13 | 258 | 42.9 |
| 14–18 | 298 | 49.6 |
| Sex | 50.3 | |
| Male | 302 | |
| Female | 299 | 49.7 |
| Educational status of household head | ||
| Illiterate | 21 | 3.4 |
| Can read and write | 104 | 17.3 |
| Primary school | 174 | 29 |
| Secondary school | 158 | 26.3 |
| College and above | 144 | 24 |
| Educational level of the child | ||
| Kindergarten | 9 | 1.5 |
| Primary school | 451 | 75 |
| Secondary school and above | 141 | 23.5 |
| Religion | ||
| Orthodox | 510 | 84.9 |
| Muslim | 67 | 11.1 |
| Protestant | 23 | 3.8 |
| Others* | 1 | 0.2 |
| Ethnicity | ||
| Amhara | 564 | 93.8 |
| Tigre | 15 | 2.5 |
| Agew | 14 | 2.3 |
| Oromo | 6 | 1.00 |
| Others** | 2 | 0.30 |
Notes: *Jehovah's witness; **Dire Dawa and Benishangul-Gumuz.
About 27.3% of children had been admitted to an NICU, and 22.1% were born prematurely (Table 2).
Table 2.
Children’s Birth History (n=601)
| Frequency | Percentage | |
|---|---|---|
| Gestational age | ||
| <37 weeks | 133 | 22.1 |
| ≥37 weeks | 468 | 77.9 |
| Mode of delivery | ||
| SVD | 542 | 90.2 |
| Cesarean section | 59 | 9.8 |
| Admission to NICU | ||
| Yes | 164 | 27.3 |
| No | 437 | 72.7 |
| Exclusive breast-feeding | ||
| Yes | 492 | 81.9 |
| No | 109 | 18.1 |
Abbreviations: SVD, spontaneous vaginal delivery; NICU, neonatal intensive-care unit.
With regard to maternal history during pregnancy, nearly 10% of study participants' mothers had had diabetes mellitus, hypertension, pneumonia, malaria, or syphilis (Table 3).
Table 3.
Maternal History During Pregnancy (n=601)
| Frequency | Percentage | |
|---|---|---|
| Maternal alcohol consumption during pregnancy | ||
| Yes | 17 | 2.8 |
| No | 584 | 97.2 |
| Maternal cigarette smoking during pregnancy | ||
| Yes | 0 | |
| No | 601 | 100 |
| Systemic illness during pregnancy | ||
| Yes* | 62 | 10.3 |
| No | 590 | 89.7 |
Note: *Hypertension, diabetes, malaria, pneumonia, syphilis.
Only eight children had had no exposure to television, while 194 watched television from ≤2 m (Table 4).
Table 4.
Socioeconomic And Behavioral Factors (n=601)
| Frequency | Percentage | |
|---|---|---|
| Family monthly income, ETB | ||
| <2,000 | 140 | 23.3 |
| 2,001–5,000 | 283 | 47.1 |
| 5,001–10,000 | 129 | 21.5 |
| 10,001–15,000 | 22 | 4.5 |
| >15,000 | 27 | 3.7 |
| Family size | ||
| <4 | 272 | 45.3 |
| 4–6 | 240 | 39.9 |
| >6 | 89 | 14.9 |
| School type | ||
| Public | 344 | 57.2 |
| Private | 257 | 42.8 |
| Medical visits (for children) | ||
| Yes | 282 | 46.9 |
| No | 319 | 53.1 |
| Frequency of medical visits (for children) | ||
| Yearly | 92 | 15.3 |
| Symptoms seen and when traumatized | 190 | 31.6 |
| History of television exposure | ||
| Yes | 593 | 98.6 |
| No | 8 | 1.40 |
| Duration of television exposure | ||
| <2 hours/day | 142 | 23.6 |
| 2–4 hours/day | 253 | 42.1 |
| >4 hours/day | 198 | 32.9 |
| Television-exposure distance | ||
| <2 m | 194 | 32.3 |
| 2–4 m | 231 | 38.3 |
| >4 m | 168 | 28.0 |
| Duration of mobile/computer exposure | ||
| <2 hours/day | 260 | 43.3 |
| 2–4 hours/day | 201 | 33.4 |
| >4 hours/day | 97 | 16.1 |
Abbreviation: ETB, Ethiopian birr.
Prevalence Of Visual Impairment
VI was diagnosed in 52 children (8.7%, 95% CI 6.2%-10.7%) 27 (52%) of whom were male. Of those children who had VI, 43 (7.2%) had moderate VI, six (1%) severe VI, and four (0.5%) were blind (Table 5).
Table 5.
Prevalence Of VI In Terms Of Age And Sex (n=601)
| Age, Years | Visual Impairment | No VI | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| 6–9 | 0 | 3 | 20 | 22 |
| 10–13 | 18 | 10 | 119 | 111 |
| 14–18 | 9 | 12 | 136 | 141 |
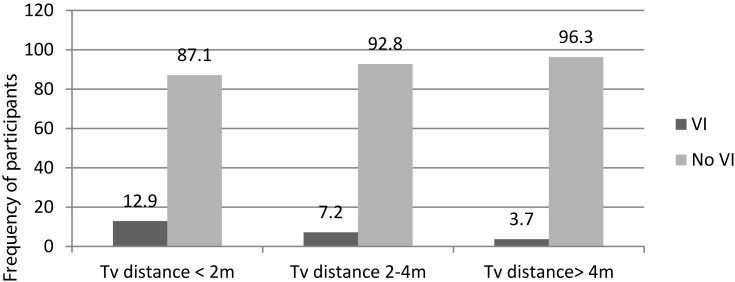
The majority of study participants were born maturely (85.2%), 82.5% had been exclusively breast-fed >6 months, 4.9% had been admitted to an NICU, and 32.3% were watching television from 2 mor closer, of which 12.9% had VI. However, 168 (28%) study participants watched television from >4 m, of which only 3.7% had VI (Figure 1).
Figure 1.
Television exposure distance characteristics of study participants in Bahir Dar, northwest Ethiopia, June 2018 (n=601).
Factors Associated With Visual Impairment
In the bivariate analysis, history of medical visits, siblings with VI, educational status of household head, exclusive breast-feeding, admission to an NICU, mode of delivery, maternal alcohol consumption, frequency of medical visits, history of systemic illness, having chronic disease (DM/Htn), parents with VI, mobile-exposure duration, gestational age, duration of television exposure, television-exposure distance, and age of the child were significantly associated with VI. However, in the multivariable logistic analysis, only five variables — admission to an NICU, prematurity (gestational age <37 weeks), children from parents with VI, television-exposure distance <2 m and mobile/computer/video-game-exposure >4 hours/day — were independently significantly associated with VI (Table 6).
Table 6.
Bivariate And Multivariate Logistic Regression Output Of Factors Associated With VI (n=601)
| Visual Impairment | COR (95% CI) | AOR (95% CI) | ||
|---|---|---|---|---|
| Yes (n=52) | No (n=549) | |||
| Age, years | ||||
| 6–9 | 7 | 42 | 1.00 | 1.00 |
| 10–13 | 24 | 230 | 1.59 (0.3–3.371) | 0.21 (0.05–0.89) |
| 14–18 | 21 | 277 | 2.19 (0.34–1.13) | 0.50 (0.20–1.04) |
| Sex | ||||
| Male | 27 | 275 | 0.92 (0.60–1.90) | |
| Female | 25 | 274 | 1.00 | |
| Educational level of household head | ||||
| Illiterate | 7 | 18 | 6.61 (0.68–11.66) | |
| Can read and write | 8 | 95 | 1.71 (0.44–6.54) | |
| Primary school | 15 | 156 | 1.44 (0.38–5.38) | |
| Secondary school | 14 | 144 | 1.75 (0.43–7.14) | |
| College and above | 8 | 136 | 1.00 | |
| Maternal illness during pregnancy | ||||
| Yes | 8 | 107 | 1.33 (1.82–22.8) | |
| No | 44 | 442 | 1.00 | |
| Type of illness during pregnancy | ||||
| Chronic disease (DM/Htn) | 22 | 24 | 2.40 (0.02–0.08) | |
| Others | 11 | 5 | 1.00 | |
| Drank alcohol during pregnancy | ||||
| Yes | 12 | 122 | 0.95 (2.25–18.04) | |
| No | 40 | 427 | 1.00 | |
| Gestational age | ||||
| <37 weeks | 22 | 249 | 1.13 (1.61–5.21) | 2.81 (1.19–6.83)*** |
| ≥37 weeks | 30 | 300 | 1.00 | 1.00 |
| Mode of delivery | ||||
| SVD | 39 | 503 | 1.00 | |
| Cesarean section | 13 | 46 | 0.27 (1.81–7.32) | |
| Admission to NICU | ||||
| Yes | 21 | 296 | 1.72 (3.56–11.64) | 5.52 (2.01–15.15)** |
| No | 31 | 253 | 1.00 | 1.00 |
| Sibling with VI | ||||
| Yes | 10 | 41 | 2.95 (1.58–6.83) | |
| No | 42 | 508 | 1.00 | |
| Parents with VI | ||||
| Yes | 16 | 247 | 1.84 (1.97–6.78) | 1.8 (0.13–0.97)* |
| No | 36 | 302 | 1.00 | 1.00 |
| Family history of spectacle use | ||||
| Yes | 19 | 54 | 5.29 (2.80–9.90) | |
| No | 33 | 495 | 1.00 | |
| History of medical visits | ||||
| Yes | 10 | 272 | 1.00 | |
| No | 42 | 277 | 0.3 (2.03–8.39) | |
| Frequency of medical visits | ||||
| Yearly | 10 | 82 | 1.00 | |
| Symptoms seen | 19 | 128 | 0.12 (0.04–0.33) | |
| Duration of watching TV | ||||
| <2 hours/day | 20 | 98 | 1.00 | |
| 2–4 hours/day | 12 | 198 | 3.36 (0.63–2.75) | |
| >4 hours/day | 6 | 155 | 5.27 (0.59–3.53) | |
| TV-exposure distance | ||||
| <2 m | 26 | 223 | 1.47 (0.69–12.81) | 8.71 (1.49–18.24)* |
| 2–4 m | 17 | 293 | 2.97 (0.86–4.87) | 2.32 (2.42–25.26) |
| >4 m | 5 | 29 | 1.00 | 1.00 |
| Duration of mobile exposure | ||||
| <2 hours/day | 20 | 245 | 1.00 | 1.00 |
| 2–4 hours/day | 12 | 154 | 1.04 (2.44–17.11) | 2.98 (1.30–6.78) |
| >4 hours/day | 6 | 99 | 1.95 (1.10–7.93) | 1.6 (1.32–4.45)* |
Notes: *p<0.05; **p<0.01; ***p<0.001.
Abbreviations: TV, television; VI, visual impairment; DM, diabetes mellitus; Htn, hypertension; SVD, spontaneous vaginal delivery; NICU, neonatal intensive-care unit.
Discussion
VI is a common ophthalmic problem in young children.3 An understanding of the prevalence of VI and associated factors adds new knowledge about the risk factors of VI, leading to better understanding and management of the condition. The overall prevalence of VI in this study was 8.7% (95% CI 6.5%–11.1%), which is in line with other studies reported across the world: (Gombak, Malaysia [7.1%]31 and Sanaa, Yemen [9.9%]),26 but higher than previous studies in Sekoru, southwest Ethiopia (0.062%),32 the whole of Ethiopia (5.3.%),33 Arada, Addis Ababa, Ethiopia (5.8%),2 Puducherry, India (6.37%),7 Ogun, Nigeria (2.09),34 Ashanti, Ghana (3.7%),35 and Sydney, Australia (2.7%).29 This discrepancy might be due to the differences in study design, study setting, and inclusion criteria. In this community-based cross-sectional study, school-age children within the community were included, while in the Addis Ababa and Sekoru studies, a school-based cross-sectional study was conducted, which missed those children having VI who stay at home due to its significant effect on learning ability. In addition to these differences, there are also economic and ethnic differences between the population of this study and other reported studies, which greatly influences VI prevalence, as reported in many studies.2,15 According to a study conducted in Nigeria, 2.09% had VI.34 This difference could be due to the fact that in this study, there was no adequate provision of eye-care services, which might have increased prevalence.
The definition of VI influences reported prevalence rates. The definition used in some studies (best-corrected visual acuity <6/18 in the better eye)2,12 differs from this study. This study used the new ICD10 definition of VI, ie, presenting distance visual acuity ≤6/18. Therefore, taking the visual acuity of the participant as present definitely increases prevalence. The prevalence found in this study was lower than in Selangor, Malaysia (25%).28 This may be due to variation in study populations. Most Asian nations are myopic because of complex genetic traits responsible for myopia, and in turn myopic cases outnumber VI cases in this population.9
This study revealed that those study participants who watched television from a distance <2 m had nearly nine times the odds of developing VI than those were watching television from >4 m, which is in agreement with a study done in Adama, Ethiopia.9 This might be related to the fact that watching television from close range creates a visual strain on the eyes of the children and increases the chance of refractive error. However, it is impossible to speculate on the temporal relationship between distance from the television and the development of VI, as myopic children move more closely tothe television to see the picture more clearly. Study participants who had a history of admission to an NICU had nearly six times the odds of developing VI than those who had had no admissions to an NICU. This might be due to phototherapy for prematurity and development of pathological jaundice in neonates, which exposes their eyes to radiation and in turn may cause VI.35
Children who had parents with VI had nearly double the odds of being visually impaired compared to those with parents who had no VI, which is in agreement with a study in southwest Nigeria.18 This is due to the inheritance nature of VI. A family history of VI is often present, but well-defined genetic patterns are unusual.6 Prematurity was associated with VI in this study. Children who were born prematurely had nearly triple the odds of being visually impaired compared to those who were born maturely. Studies from Denmark14–21 and Australia17 support this result. This could be due to risk of refractive error in premature infants and prematurely developed sensory and motor systems, which could be associated with VI.
The results we found were more extreme than other studies, and this is significant for public health. This implies that the prevention techniques applied were at a low level. This might be related to poor utilization of eye-care services and the limitation of technologically advanced health-care services. According to the Vision 2020 plan, the major priority area to act on is elimination of avoidable VI.36 To achieve the goal, a regular school screening program and community service is required.
Limitations
Assessment of VI was done with visual acuity only, which did not include visual field testing. The majority of questions in the data-collection instrument asked about subject history, and would have been exposed to recall bias. As this study was cross-sectional study, it was impossible to identify which comes first: the associated factors or the outcome. For example, according to this study, watching television from <2 m may cause a visual strain and development of myopia, and logically myopic children move more closely to the television, so it is impossible to determine which comes first. Causality and such temporal relationships between dependent and other independent variables were not clearly identified either, due to the design of the study. In order to identify such temporal relationships clearly, we recommend other studies be conducted with better design.
Conclusion
The prevalence of VI among school-age children in Bahir Dar was 8.7%, which is higher than other studies. Moderate VI was most common among visually impaired children. Premature birth, admission to NICU, parents with VI, television exposure <4 m, and mobile-exposure duration >2 and 4 hours per day were independently significantly variables associated with VI.
Acknowledgments
We are deeply indebted to the University of Gondar, which gave ethical clearance to conduct this research. We would also like to acknowledge the study participants for their cooperation and willingness to participate in this study.
Availability Of Data
The hard copy of the collected primary data used in this research is securely locked so that is accessible to the authors only. For the sake of privacy of the participants, the data are not totally open to all readers; however, data can be made available from one of the authors upon reasonable request.
Disclosure
The authors report no conflicts of interest with respect to the research, authorship, or publication of this article.
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Darge HF, Shibru G, Mulugeta A, et al. The prevalence of visual acuity impairment among school children at Arada Subcity primary schools in Addis Ababa, Ethiopia. J Ophthalmol. 2017;2017:9326108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–618. doi: 10.1136/bjophthalmol-2011-300539 [DOI] [PubMed] [Google Scholar]
- 4.Resnikoff S, Pascolini D, SP Mariotti, et al. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull World Health Organ. 2008;86(1):63–70. doi: 10.2471/BLT.00.000000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demissie BS, Solomon AW. Magnitude and causes of childhood blindness and severe visual impairment in Sekoru District, Southwest Ethiopia: a survey using the key informant method. Trans R Soc Trop Med Hyg. 2011;105(9):507–511. doi: 10.1016/j.trstmh.2011.04.007 [DOI] [PubMed] [Google Scholar]
- 6.Weingeist T, Liesegang T, Grand M. American academy of ophthalmology, basic and clinical science course 2000–2001 lens and cataract. Biochemistry. 10–17. [Google Scholar]
- 7.Vishnuprasad R, Bazroy J, Madhanraj K, et al. Visual impairment among 10–14-year school children in Puducherry. J Family Med Prim Care. 2017;6(1):58. doi: 10.4103/2249-4863.214983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aniza I, AM Nawi, Jamsiah M, et al. Prevalence of visual acuity impairment and its associated factors among secondary school students in Beranang, Selangor. Malaysian J Publ Health Med. 2012;12(1):39–44. [Google Scholar]
- 9.Bezabih L, Abebe TW, Fite RO. Prevalence and factors associated with childhood visual impairment in Ethiopia. Clin Ophthalmol. 2017;11:1941. doi: 10.2147/OPTH.S135011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston P, McCarty C, Taylor H. Visual impairment and socioeconomic factors. Br J Ophthalmol. 1997;81(7):574–577. doi: 10.1136/bjo.81.7.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faal H, Minassian D, Sowa S, et al. National survey of blindness and low vision in The Gambia: results. Br J Ophthalmol. 1989;73(2):82–87. doi: 10.1136/bjo.73.2.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitfield R, Schwab L, Ross-Degnan D, et al. Blindness and eye disease in Kenya: ocular status survey results from the Kenya Rural Blindness Prevention Project. Br J Ophthalmol. 1990;74(6):333–340. doi: 10.1136/bjo.74.6.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pai AS-I, Wang JJ, Samarawickrama C, et al. Prevalence and risk factors for visual impairment in preschool children: the Sydney Paediatric Eye Disease Study. Ophthalmology. 2011;118(8):1495–1500. doi: 10.1016/j.ophtha.2011.01.027 [DOI] [PubMed] [Google Scholar]
- 14.Robaei D, Rose KA, Kifley A, Cosstick M, Ip JM, Mitchell P. Factors associated with childhood visual impairment: findings from a population-based study. Ophthalmology. 2006;113(7):1146–1153. doi: 10.1016/j.ophtha.2006.02.019 [DOI] [PubMed] [Google Scholar]
- 15.Blencowe H, Lawn JE, Vazquez T, et al. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74(S1):35. doi: 10.1038/pr.2013.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torp-Pedersen T, Boyd HA, Poulsen G, et al. Perinatal risk factors for visual impairment. Int J Epidemiol. 2010;39(5):1229–1239. doi: 10.1093/ije/dyq092 [DOI] [PubMed] [Google Scholar]
- 17.Azonobi I, et al. Risk factors for visual impairment in Southwestern Nigeria. Pak J Ophthalmol. 2009;25:3. [Google Scholar]
- 18.Chia A, Lin X, Dirani M, et al. Risk factors for strabismus and amblyopia in young Singapore Chinese children. Ophthalmic Epidemiol. 2013;20(3):138–147. doi: 10.3109/09286586.2013.767354 [DOI] [PubMed] [Google Scholar]
- 19.Gilbert CE, Shah SP, Jadoon MZ, et al. Poverty and blindness in Pakistan: results from the Pakistan national blindness and visual impairment survey. Bmj. 2008;336(7634):29–32. doi: 10.1136/bmj.39395.500046.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster A, Gilbert C. Epidemiology of childhood blindness. Eye. 1992;6(2):173. doi: 10.1038/eye.1992.34 [DOI] [PubMed] [Google Scholar]
- 21.Powls A, Botting N, Cooke RWI, et al. Visual impairment in very low birthweight children. Arch Dis Child Educ Pract Ed. 1997;76(2):F82–F87. doi: 10.1136/fn.76.2.F82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goujon A, Barakat B, Goujon A, et al. Projection of populations by level of educational attainment, age, and sex for 120 countries for 2005–2050. Demogr Res. 2010;22:383–472. doi: 10.4054/DemRes.2010.22.15 [DOI] [Google Scholar]
- 23.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. BMC. 2005;30/2006:24–41. [DOI] [PubMed] [Google Scholar]
- 24.Macnaughton J. Eye Essentials: low Vision Assessment. Clin Exp Optom. 2006;89(2):118–120. [Google Scholar]
- 25.WHO, Change the definition of blindness. WHO, 2008.35. Available From: www.who.int›blindness›ChangetheDefinitionofBlindness Accessed October14, 2019
- 26.Bamashmus M, Al-Akily S. Profile of childhood blindness and low vision in Yemen: a hospital-based study/Profil de la cecite et de la basse vision chez l’enfant au Yemen: une etude hospitaliere. East Mediterr Health J. 2010;16(4):425. doi: 10.26719/2010.16.4.425 [DOI] [PubMed] [Google Scholar]
- 27.Tielsch JM, Katz J, Sommer A, et al. Family history and risk of primary open angle glaucoma: the Baltimore Eye Survey. Arch Ophthalmol. 1994;112(1):69–73. doi: 10.1001/archopht.1994.01090130079022 [DOI] [PubMed] [Google Scholar]
- 28.Edussuriya K, Sennanayake S, Senaratne T, et al. The prevalence and causes of visual impairment in central Sri Lanka the Kandy Eye study. Ophthalmology. 2009;116(1):52–56. doi: 10.1016/j.ophtha.2008.08.034 [DOI] [PubMed] [Google Scholar]
- 29.Kaminski M, Rumeau C, Schwartz D. Alcohol consumption in pregnant women and the outcome of pregnancy. Alcohol Clin Exp Res. 1978;2(2):155–163. doi: 10.1111/j.1530-0277.1978.tb04716.x [DOI] [PubMed] [Google Scholar]
- 30.Goh PP, Abqariyah Y, Pokharel GP, Ellwein LB. Refractive error and visual impairment in school-age children in Gombak District, Malaysia. Am Acad Ophthalmol. 2004;56(0161–6420/05). [DOI] [PubMed] [Google Scholar]
- 31.Demissiea BS, Solomon AW. Solomon, magnitude and causes of childhood blindness and severe visual impairment in Sekoru District, Southwest Ethiopia. Royal Soc Trop Med Hyg. 2010;105:507–511 [DOI] [PubMed] [Google Scholar]
- 32.Berhane Y, Worku A, Bejiga A, et al.. Ethiop J Health Dev. 2007;21(3):204–210. [Google Scholar]
- 33.Fasina F, Ajaiyeoba A. The prevalence and causes of blindness and low vision in Ogun State, Nigeria. Afr J Biomed Res. 2003;6(2). [Google Scholar]
- 34.Kumah BD, Ebri A, Abdul-Kabir M, et al. Refractive error and visual impairment in private school children in Ghana. Am Acad Optometry. 2013;90(12):1456–1461. doi: 10.1097/OPX.0000000000000099 [DOI] [PubMed] [Google Scholar]
- 35.Wu PY, Lim RC, Hodgman JE, et al. Effect of phototherapy in preterm infants on growth in the neonatal period. J Pediatr. 1974;85(4):563–566. doi: 10.1016/S0022-3476(74)80471-3 [DOI] [PubMed] [Google Scholar]
- 36.WHO. Global initiative for the elimination of avoidable blindness: action plan 2006–2011. 2007. Available from: https://apps.who.int/iris/handle/10665/43754 Accessed October11, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- WHO, Change the definition of blindness. WHO, 2008.35. Available From: www.who.int›blindness›ChangetheDefinitionofBlindness Accessed October14, 2019
Data Availability Statement
The hard copy of the collected primary data used in this research is securely locked so that is accessible to the authors only. For the sake of privacy of the participants, the data are not totally open to all readers; however, data can be made available from one of the authors upon reasonable request.