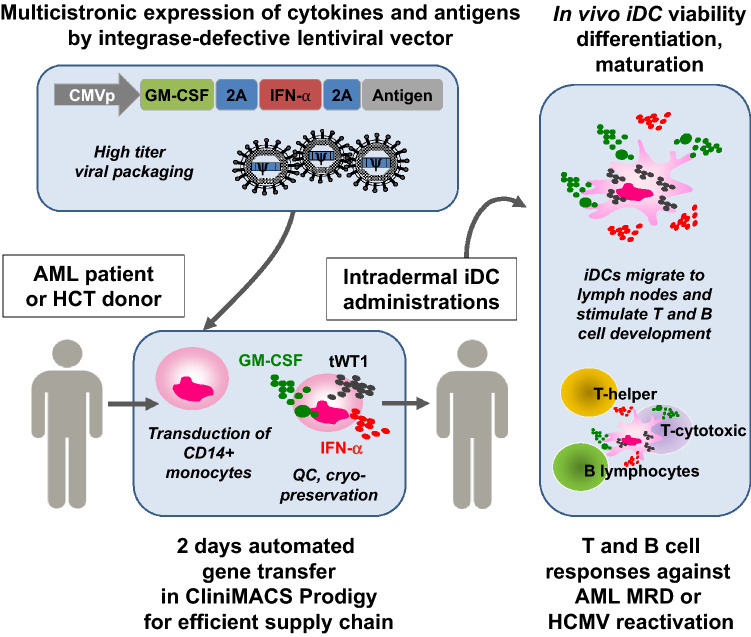
Fig. 1.
Schematic representation of the tricistronic integrase-defective lentiviral vector design and manufacturing process of reprogrammed monocytes that self-differentiate into induced dendritic cells (iDC). CD14+ monocytes obtained from the AML patient or HSCT donor are enriched and transduced with high titer lentiviral vector in a CliniMACS Prodigy automated system. After quality control, the cryopreserved iDC cell product can be stored or shipped to other clinical centers. After thawing of the product and intradermal administration into patients, highly viable self-differentiated iDC will secrete GM-CSF and IFN-α2 and process antigens, stimulating the development of functional T and B cell responses. The combined adaptive responses will protect patients against AML relapse (tWT1 antigen) or HCMV reactivation (HCMV antigens pp65/gB)