Abstract
Background: Breast cancer rates have been increasing worldwide, particularly among young women, suggesting important interactions between genes and health behaviors. At the same time, mobile technology, including smartphones applications (apps), has emerged as a new tool for delivering healthcare and health-related services. As of 2018, there were nearly 600 publicly available breast cancer apps designed to provide disease and treatment information, to manage disease, and to raise overall awareness. However, the extent to which apps are incorporated into breast cancer prevention research is unknown. Therefore, the objective of this review was to determine how mobile applications are being used for breast cancer prevention among women across the cancer control continuum.
Methods: Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we searched PubMed and Web of Science Core Collection databases using the keywords breast cancer, smartphone, mobile application, and phone app. Full-length journal articles available in English that addressed the research question were included. We categorized articles by prevention type (primary, secondary, and tertiary) and phase of research (protocol, development, feasibility, pilot, measurement, and effectiveness), and identified common themes and gaps.
Results: Our search yielded 82 studies (69 unique) that used apps in breast cancer prevention research across 20 countries. Approximately half of the named apps were publicly available. The majority (73%) of studies targeted tertiary prevention; 15% targeted secondary and 13% targeted primary prevention. Apps were used across all phases of research with the predominant phase being feasibility in tertiary prevention (34%), effectiveness in secondary prevention (63%), and development (30%) and effectiveness (30%) in primary prevention. Common uses included assessing outcomes relevant to clinical care coordination, quality of life, increasing self-efficacy and screening behaviors, and tracking and managing health behaviors.
Conclusions: We identified the following gaps: few effectiveness studies in tertiary prevention, minimal use of apps for breast cancer etiology or early detection, and few interventions in those at average risk of breast cancer. These findings suggest that while mobile apps can inform breast cancer prevention across the continuum, more work is needed to incorporate apps into primary prevention.
Keywords: breast cancer, cancer control continuum, mobile application, smartphone, prevention, systematic review
Introduction
Breast cancer rates have been increasing worldwide, particularly among young women (1). Such rapid changes in the incidence of early onset breast cancer cannot be attributed solely to genetics, but rather to interactions between health behaviors and genes. Given many behavioral risk factors for breast cancer are modifiable, public health prevention and intervention studies have long sought to change individual health behaviors and more recent work recognizes that a multi-faceted approach is needed to address these behaviors because they are complex in nature (2).
At the same time, mobile technologies, including smartphone applications (hereafter referred to as apps), have emerged as new tools for delivering healthcare and health-related services in the field of cancer and particularly breast cancer. In fact, nearly half of all cancer apps are targeted toward breast cancer (3). A recent review suggests there are nearly 600 publicly available breast cancer apps designed to provide disease and treatment information, to manage disease, and to raise overall awareness (4). With the widespread availability and use of applications, researchers have an opportunity to leverage this ubiquitous technology for breast cancer prevention. However, the extent to which apps are incorporated into breast cancer prevention research across the cancer control continuum is unknown.
Given that the use of apps for breast cancer prevention is still in the early stages of adoption, the authors agreed that a systematic review with a broad research scope was warranted. Therefore, we performed a systematic review to answer the question: how are mobile apps being used for breast cancer prevention research across the cancer control continuum, including tertiary, secondary, and primary prevention, in women? Since the use of apps in research is relatively new, we also sought to identify at what phases of the research process mobile apps were being used for breast cancer research, including protocol, development, feasibility, pilot, effectiveness, and measurement studies. In addition to the systematic review, we sought to find common themes and gaps across the body of literature.
Methods
Search Strategy
In order to conduct this systematic review, we utilized the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (5). We systematically reviewed PubMed and Web of Science Core Collection databases in December 2018 (updated February 7, 2019 to ensure the most recent articles were captured). Search terms included breast cancer, smartphone, mobile application, and phone app. These terms were applied to all fields in order to capture the greatest number of articles. We also employed the controlled vocabulary of Medical Subject Headings (MeSH), available in PubMed only, including subheadings, for breast neoplasms and mobile apps. Supplementary Table 1 includes the complete search string as it was conducted in PubMed. We searched for additional articles using the terms mHealth, health app, breast cancer app, iPhone application, and Android application. Our search contained no restrictions regarding language or year of publication. All references were exported to Endnote (X8, Thompson Reuters). We first removed duplicate citations using the automatic feature and then manually reviewed articles for additions that had minor differences in the way information was indexed.
Inclusion/Exclusion Criteria
Records were screened in Endnote and included if they were published as an original research article in English. The primary reviewer [RH] then reviewed the full-text article for relevance to the study question. Articles were excluded if study participants were providers or caregivers; if breast cancer prevention was not an explicit goal or implication of the research; if the article did not include a mobile application or only discussed that the research could be potentially adapted into a mobile application; or if the smartphone was examined as a carcinogen. We also excluded books or book chapters, meeting abstracts, non-empirical records (e.g., reviews, editorials, and letters), non-English records, and records where the full-text were unavailable. When inclusion was unclear, authors LH and JAM independently reviewed the articles and then all authors discussed until a consensus was met. LH and JAM also reviewed 20% of excluded articles for accuracy. In one case where we could not reach consensus, we contacted the corresponding author for clarification. Among all studies that were eligible for qualitative analysis (n = 82), we flagged those studies that had multiple publications reporting outcomes across different stages of research (e.g., a protocol and effectiveness study) but were using the same underlying cohort (n = 23).
Data Extraction and Analysis
For studies meeting the inclusion criteria, the primary reviewer [RH] extracted the following information from eligible studies: population characteristics, sample size, location of the study (country), mobile application name (where applicable), and study objectives and/or outcomes (e.g., quality of life, efficacy, literacy). We categorized studies by prevention type based on whether they were targeting a secondary cancer event and/or morbidity/mortality (tertiary), early diagnosis and treatment (secondary), or disease prevention (primary). We assigned articles to only one prevention type category. We also categorized studies by research phase based on the study outcome(s). Studies categorized as Development included those collecting information on participant interest and preferences for a mobile application that was not yet produced. Based on features outlined by Orsmond and Cohn (6), we categorized Feasibility studies as those that reported process outcomes, such as usability of an app (6). We categorized Pilot studies as those studies where the author(s) self-described the study as such and/or the authors(s) mention that a larger study was being planned to evaluate the effectiveness of an intervention. Generally, Pilot studies reported outcomes among a small sample, where the average sample size was ~35. Effectiveness studies reported outcome measures from a full study; and a Protocol described the protocol for a study, such as for an effectiveness study, usually in the title of the article itself. Measurement studies were those that reported outcomes related to validity or reliability. Some studies were categorized across multiple research phases if papers combined multiple outcomes; therefore, research phase categories were not mutually exclusive.
Our initial analysis tabulated all articles eligible for qualitative analysis by cancer prevention type and by research phase. We then estimated the number of articles published by year. We used the subset of unique studies and tabulated the number of publications by country and continent. Lastly, void of a priori hypotheses regarding common themes and gaps in the literature, we comprehensively reviewed unique studies by cancer prevention type to identify common themes and gaps. We then extracted mobile app details and categorized app use by prevention type and the availability of the app in the Apple and/or Android app store.
Results
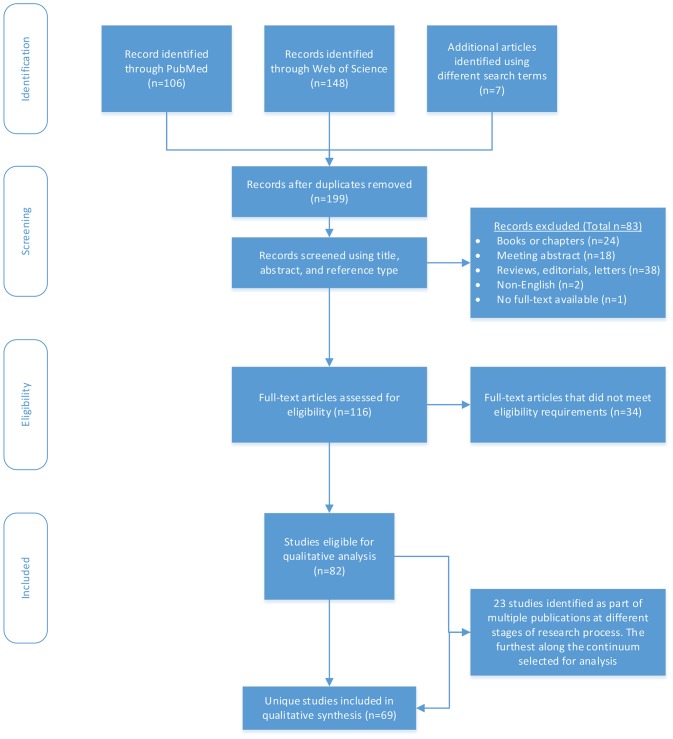
We identified 199 records through our search, excluding duplicate records (Figure 1). Of these, we first screened the record title, abstract, and reference type for eligibility and excluded 83 records as ineligible. We then assessed the remaining 116 articles for eligibility through full-text review and further excluded 34 records. We identified 82 studies eligible for qualitative analysis. Of the 82, we identified 23 studies that were part of multiple publications that used the same underlying cohort to report outcomes across different research phases. Therefore, we identified 69 unique studies, 75% (n = 52) were tertiary, 12% (n = 8) were secondary, and 13% (n = 9) were primary.
Figure 1.
Flow chart of systematic review.
The Use of Mobile Apps by Cancer Prevention Type and Research Phase
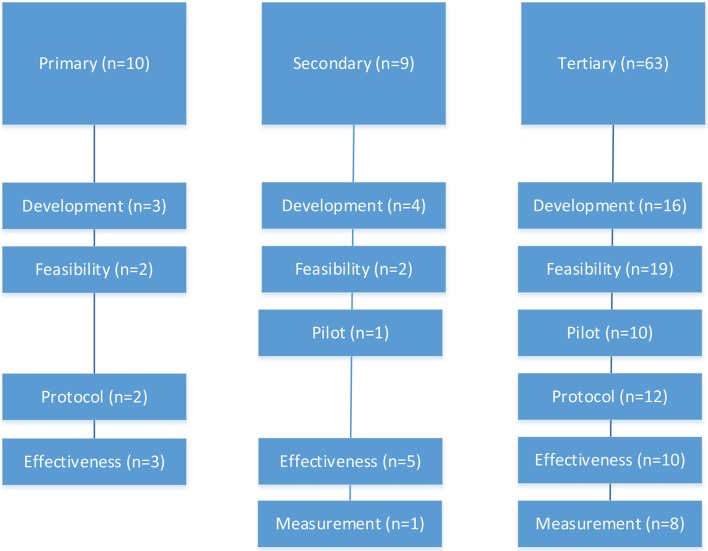
As displayed in Figure 2, apps were used across all phases of research with the predominant phase being feasibility in tertiary prevention studies (34%), effectiveness in secondary prevention studies (63%), and development (30%) and effectiveness (30%) in primary prevention studies. Across the cancer prevention continuum, 14 studies were protocols (17%), 23 were development (28%), 23 were feasibility (28%), 11 were pilots (13%), 18 were effectiveness (22%), and 9 were measurement studies (11%). Given 23 articles reported on multiple study phases, the categories were not mutually exclusive and percentages exceed 100%.
Figure 2.
The use of mobile apps across primary, secondary, and tertiary breast cancer prevention by research phase (n = 82 eligible studies).
Mobile App Use: Growth and Global Reach
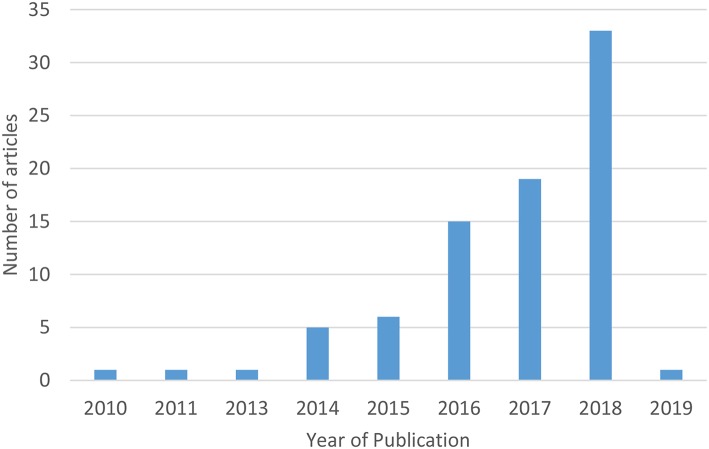
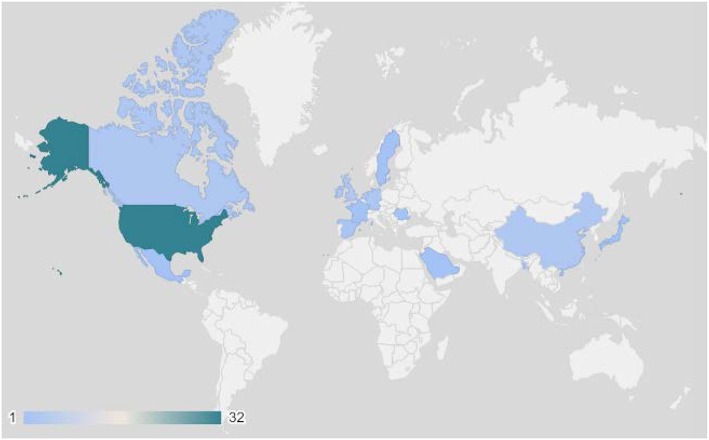
The number of studies using apps for breast cancer prevention research increased rapidly over the last 10 years (Figure 3). The earliest studies in this review were published in 2010, while the majority (40%) were published in 2018. There was international use of apps in breast cancer prevention research, with the exception of Africa and South America (Figure 4). The studies included in this review were conducted in 20 countries, with most studies conducted in the US (43%) and more than one study each occurring in Canada (7–9), China (10–12), Germany (13–15), Ireland (16–18), Korea (19–24), the Netherlands (25–29), Spain (30, 31), and the United Kingdom (32–35). Tertiary prevention studies took place in North America (US, Canada, Mexico), Western Europe (UK, Sweden, Netherlands, Germany, France, Spain Ireland), and Asia (Korea, China, Japan, Singapore). Secondary prevention studies were based in North America (US), Asia (Korea, China, India, Bangladesh), and Eastern Europe (Romania). Primary prevention studies were based in North America (US), Europe (Netherlands), and the Middle East (Kingdom of Saudi Arabia).
Figure 3.
Number of studies using mobile apps for breast cancer prevention research among women by year of publication (n = 82 eligible studies). *The initial search was conducted in December 2018 and updated February 7, 2019.
Figure 4.
Number of publications by country (n = 69 unique studies).
Review of Mobile Apps by Cancer Prevention Types: Common Themes
Tertiary Prevention
The majority of mobile apps used for breast cancer prevention research addressed tertiary prevention. We identified 63 studies (53 unique) (Table 1) and the articles ranged across research phases including development (24.5%), feasibility with a focus on process (34%), pilots with a focus on outcomes (18.9%), protocols (15.1%), effectiveness (16%), and measurement (11.3%) (Figure 2).
Table 1.
Articles using mobile apps for tertiary breast cancer prevention (n = 63 eligible studies).
| References | Type of study | Population (sample size) | Location | Outcomes |
|---|---|---|---|---|
| Ainsworth et al. (36) | Feasibility | Breast cancer survivors (40) | US | App use and experience |
| Akechi et al. (37) | Protocol | Breast cancer survivors (444) | Japan | Fear of recurrence |
| Ali et al. (38) | Development | Patients undergoing treatment for cancer (423) | Singapore | App interest and preferences |
| Armstrong et al. (8) | Effectiveness | Women undergoing breast reconstruction (65) | Canada | Post-surgical follow-up |
| Armstrong et al. (39)* | Protocol | Women undergoing breast reconstruction (72) | Canada | Post-surgical follow-up |
| Banas et al. (40) | Development | Breast cancer survivors, Hispanic (31) | US | App interest and preferences |
| Baseman et al. (41) | Feasibility | Breast cancer survivors and providers (11) | US | App interest and preferences |
| Brett et al. (34) | Development | Women undergoing treatment for breast cancer (20) | UK | App use and experience |
| Buscemi et al. (42) | Feasibility + Pilot | Breast cancer survivors, Hispanic (25) | US | App use and experience, Quality of life |
| Iacobelli et al. (43)* | Development | Breast cancer survivors, Hispanic (9) | US | App interest and preferences |
| Yanez et al. (44)* | Protocol | Breast cancer survivors, Hispanic (80) | US | Quality of life |
| Chalela et al. (45) | Protocol | Women undergoing treatment for breast cancer (120) | US | Medication adherence |
| Delrieu et al. (46) | Protocol | Women undergoing treatment for breast cancer (60) | France | Physical activity, app use |
| Douma et al. (28) | Feasibility + Measurement | Women undergoing treatment for breast cancer (72) | Netherlands | Physical activity, app use |
| Drewes et al. (13) | Development | Women undergoing treatment for breast cancer and physicians (168) | Germany | App interest and preferences |
| Egbring et al. (14) | Effectiveness | Women undergoing treatment for breast cancer (139) | Germany | Daily functional activity |
| El Shafie et al. (15) | Development | Patients undergoing treatment for cancer (breast or prostate) (200) | Germany | App interest and preferences |
| Foley et al. (17) | Pilot | Women undergoing treatment for breast cancer (39) | Ireland | Mental health |
| Gehrke et al. (47) | Development + Feasibility | Breast cancer survivors (11) and their nurses (3) | US | App interest and preferences |
| Harder et al. (33) | Development + Feasibility | Women undergoing treatment for breast cancer (9) | UK | App interest and preferences |
| Hwang (7) | Effectiveness | Women undergoing treatment for breast cancer (72) | Canada | Readmission, app use and experience |
| Kim et al. (23) | Effectiveness | Women undergoing treatment for breast cancer (76) | Korea | Medication adherence |
| Kim et al. (21) | Measurement | Women undergoing treatment for breast cancer (78) | Korea | Reliability |
| Klasnja et al. (48) | Effectiveness | Women undergoing treatment for breast cancer (9) | US | Self-management |
| Klasnja et al. (49)* | Development | Women undergoing treatment for breast cancer (3) | US | App interest and preferences |
| Kubo et al. (50) | Feasibility + Pilot | Patients undergoing treatment for cancer (28) and their caregivers (14) | US | App use and experience, distress and quality of life |
| Langer et al. (51) | Measurement | Women undergoing treatment for breast cancer and their partners (107 couples) | US | Relationship satisfaction |
| Langius-Eklof et al. (52) | Protocol | Patients undergoing treatment for cancer (150) | Sweden | Symptom distress |
| Lloyd et al. (53) | Development | Breast cancer survivors (279) | US | App interest and preferences |
| Lozano-Lozano et al. (30) | Protocol | Breast cancer survivors (80) | Spain | Quality of life |
| Lozano-Lozano et al. (54)* | Measurement | Breast cancer survivors (20) | US | Validity and test-retest reliability |
| Lyons et al. (55) | Protocol | Breast cancer survivors (120) | US | Physical activity |
| McCarroll et al. (56) | Pilot | Breast and endometrial cancer survivors (50) | US | Physical activity |
| Min et al. (20) | Feasibility | Women undergoing treatment for breast cancer (30) | Korea | App use and experience |
| O'Brien et al. (16) | Development | Breast clinic sample (200) | Ireland | App use and experience |
| Ormel et al. (29) | Feasibility + Pilot | Patient undergoing treatment for cancer or cancer survivors (32) | Netherlands | Physical activity, use and experience |
| Paredes-Aracil et al. (57) | Measurement | Breast cancer survivors (272) | Spain | Model validation and calibration |
| Paredes-Aracil et al. (31)* | Measurement | Breast cancer survivors (287) | Spain | Model validation and calibration |
| Park et al. (24) | Effectiveness | Women undergoing treatment for breast cancer (356) | Korea | Physical activity |
| Lee et al. (58)* | Feasibility | Breast cancer survivors (88) | Korea | App use and experience |
| Phillips et al. (59) | Protocol | Breast cancer survivors (256) | US | Physical activity, use and experience |
| Phillips et al. (59) | Feasibility | Breast cancer survivors (279) | US | App interest and preferences |
| Pope et al. (60) | Feasibility + Pilot | Breast cancer survivors (10) | US | Physical activity, use and experience |
| Quintiliani et al. (61) | Feasibility + Pilot | Breast cancer survivors (10) | US | App use and experience, weight management |
| Raghunathan et al. (62) | Development | Patients undergoing cancer treatment (631) | US | App interest and preferences |
| Ritvo et al. (9) | Protocol | Breast cancer survivors (107) | Canada | Physical activity, use and experience |
| Roberts et al. (35) | Development | Cancer survivors (breast, prostate, colorectal) (32) | UK | App interest and preferences |
| Rosen et al. (63) | Feasibility + Effectiveness | Breast cancer survivors (112) | US | Quality of life, use and experience |
| Smith et al. (64) | Development | Breast cancer survivors, African American (96) | US | App interest and preferences |
| Soto-Perez-De-Celis et al. (65) | Pilot + Feasibility | Patients undergoing cancer treatment (40) | Mexico | Physical activity, use and experience |
| Stubbins et al. (66) | Effectiveness | Breast cancer survivors (33) | US | Weight management |
| Timmerman et al. (25) | Measurement | Cancer survivors (18) | Netherlands | Physical activity, reliability |
| Uhm et al. (22) | Effectiveness | Breast cancer survivors (356) | Korea | Physical activity |
| Valle et al. (67) | Feasibility + Pilot | Breast cancer survivors, African American (35) | US | Weight management and physical activity |
| Walker et al. (68) | Development | Breast cancer survivors and nurses (12) | US | App use and experience |
| Weaver et al. (32) | Pilot | Patients undergoing treatment for cancer (breast or colorectal) (26) | UK | Medication use and perceived support |
| Xiaosheng et al. (11) | Protocol | Breast cancer survivors (60) | China | Quality of life |
| Young-Afat et al. (27) | Feasibility | Women undergoing treatment for breast cancer (15) | Netherlands | App use and experience |
| Zhang et al. (69) | Feasibility | Cancer survivors and workshop attendees (~150) | Europe | App use and experience |
| Zhu et al. (70) | Effectiveness | Women undergoing treatment for breast cancer (114) | China | Self-efficacy |
| Zhu et al. (12)* | Feasibility | Women undergoing treatment for breast cancer (13) | China | App use and experience |
| Zhu et al. (71)* | Protocol | Women undergoing treatment for breast cancer (108) | China | Self-efficacy |
| Zhu et al. (71)* | Development | Women undergoing treatment for breast cancer (114) | China | Quality of life |
Duplicate articles are indented.
US, United States; UK, United Kingdom.
We identified two common themes for the use of mobile health apps in tertiary breast cancer prevention: clinical care coordination and health related quality of life during and after a breast cancer diagnosis. Cancer care coordination studies focused on the support and communication between the breast cancer patient and the physician (32, 41, 47, 48, 66, 68), as well as specific aspects of cancer care coordination, such as symptomology (12, 14, 23, 27, 52), medication adherence (23, 34, 38, 45, 66), and ambulatory surgery (7, 8). Research using apps designed to improve health related quality of life focused on general lifestyle management (30, 42, 56, 60, 64, 69), weight management (61, 66, 67), depression and breast cancer related distress (12, 17, 21, 23, 37, 63), social support (12, 40, 50, 51), sleep (20), and physical activity during and after a breast cancer diagnosis (9, 11, 22, 24, 25, 28, 29, 33, 35, 36, 46, 55, 59, 65). The use of mobile apps for tertiary cancer prevention was preferred in contrast to usual standard of care practices. For example, multiple studies reported that cancer patients and survivors were willing, and had a preference for, receiving clinical care coordination support (13, 15, 16) and health-related quality of life interventions (53, 62) through apps.
In addition to the two main themes identified, we also found that tertiary prevention apps were used to improve measurement and provide real-time data for assessment and prediction. For example, Timmerman et al. subjectively measured fatigue in 18 cancer survivors by administering the Visual Analog Scale on a smartphone 3 times per day (25). In addition, Langer et al. had cancer patient and spouse dyads systematically record their thoughts via a smart phone twice a day for 14 consecutive days to assess communication (51). Information collected from mobile apps was also validated against other metrics. For instance, Kim et al. found that daily self-reported depression ratings collected by a mobile mental-health application provided comparable results as traditional one-time in-clinic assessment of depression and that higher accuracy of depression was achieved with greater adherence to mobile app use (21). Lastly, information collected via mobile applications was utilized to improve prediction of breast cancer-specific mortality and breast cancer recurrence (31, 57). While risk modeling is a common tool used in clinical practice to inform individuals of their individual cancer risk, Parades-Aracil et al. integrated these risk models into an app making the risk measurement tool more accessible for clinical use.
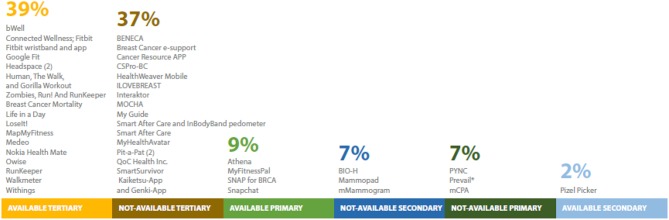
The vast majority of the apps we identified for clinical care coordination were not named in the study or publicly available, but rather developed for each specific study. In contrast, studies using apps to improve health related quality of life were more readily available for public use in the Apple and/or Android app store (Figure 5).
Figure 5.
Names and number of publicly-available apps used for breast cancer prevention research (n = 69 unique studies). Twenty-one studies excluded because no app name was provided or no app was developed. *Name provided at request of author.
Secondary Prevention
We identified 9 studies (8 unique) that used apps for secondary breast cancer prevention in the following phases: development (37.5%), feasibility (25%), pilot (12.5%), and effectiveness (62.5%); with three articles reporting on multiple study phases (see Table 2).
Table 2.
Articles using mobile apps for secondary breast cancer prevention (n = 9 eligible studies).
| References | Type of study | Population (sample size) | Location | Outcomes |
|---|---|---|---|---|
| Cardos et al. (72) | Feasibility | Community sample of females (16) | Romania | App use and experience |
| Eden et al. (73) | Pilot + Effectiveness | Clinic sample of females (100) | US | Decisional conflict and intention to screen |
| Ginsburg et al. (74) | Effectiveness | Women with abnormal clinical breast examination (556) | Bangladesh | Adherence to screening |
| Heo et al. (19) | Development + Effectiveness | Community sample of females (45) | Korea | Adherence to screening |
| Jiao et al. (10) | Development | N/A | China | Colorimetric detection of breast cancer cells |
| Keohane et al. (18) | Effectiveness | Breast clinic sample (84) | Ireland | Knowledge of risk |
| Lee et al. (75) | Effectiveness + Feasibility | Community sample, Korean American women (120) | US | Knowledge and adherence to screening; app use and experience |
| Lee et al. (58)* | Development | Community sample, Korean American women (14) | US | App interest and preferences |
| Tewary et al. (76) | Development + Measurement | Breast cancer tissue samples (30) | India | Automated Ki67 proliferation index scoring |
Duplicate articles are indented.
US, United States.
We identified only one theme in the studies of secondary prevention; with one exception (72), all studies that involved human subjects were effectiveness studies that targeted breast cancer screening behaviors, especially among underserved populations and high-risk women (18, 19, 73–75). For example, Eden et al. found that among rural women aged 40–49 years, apps were effective at reducing decisional conflict and increasing self-efficacy around mammography (73). Two studies used mobile apps to increase breast-screening practices in Korean women. Heo et al. successfully introduced an app to increase breast self-examination among young Korean women (average 29.5 ± 5.9 years) (19). In addition, Lee et al. found that in comparison to the usual care control group that received a printed brochure, Korean American women in the intervention group that received access to a mobile mammography app with health navigator services, showed significantly increased knowledge of breast cancer and greater readiness for mammography (75). Similar to Lee et al., other studies also examined if breast cancer screening is improved when pairing mobile apps with community health navigators (18, 74).
Two developmental studies used apps to innovate breast cancer detection strategies. The SmartIHC-Analyzer mobile app automates scoring of Ki-67 protein, a hallmark for assessing cell proliferation rate during cancer progression (76). The Pixel Picker mobile app rapidly detects breast cancer cells (10).
With one exception (10), none of the mobile apps for secondary prevention were publicly available at the time of this review (Figure 5).
Primary Prevention
We identified 10 articles (9 unique) that focused on the use of mobile apps for primary breast cancer prevention (see Table 3). The articles ranged across the following research phases: development (30%), feasibility (20%), protocols (20%), and effectiveness (30%).
Table 3.
Articles using mobile apps for primary breast cancer prevention (n = 10 eligible studies).
| References | Type of study | Population (sample size) | Location | Outcomes |
|---|---|---|---|---|
| Alanzi et al. (77) | Effectiveness | Community sample of female students (200) | Kingdom of Saudi Arabia | Breast cancer awareness; Guidelines; High-risk; |
| Businelle et al. (78) | Effectiveness | Hospital sample (92) | US | Smoking lapse; High-risk |
| Cohen et al. (79) | Feasibility | Community sample of females with BRCA mutation (102) | US | Awareness; Guidelines |
| Scherr et al. (80)* | Development | Community sample of females with BRCA mutation (14) and healthcare providers who work with BRCA carriers (3) | US | App preferences; Framework |
| Coughlin et al. (81) | Development | Community sample (5) | US | App preferences; Framework; Literacy |
| Hartman et al. (82) | Effectiveness | Breast clinic sample (54) | US | Weight gain and physical activity; High-risk; Framework |
| Kratzke et al. (83) | Development | Community sample of female students (546) | US | App preferences; Framework; Self-efficacy |
| Loef et al. (26) | Protocol | Healthcare workers (1960) | Netherlands | Infection susceptibility; High-risk |
| Smith et al. (64) | Protocol | Breast cancer survivors, African American (12) | US | App preferences; Guidelines; Framework |
| Bravo et al. (84) | Feasibility | Breast clinic sample (15) | US | Acceptability and usability; Literacy |
Duplicate articles are indented.
US, United States.
We identified three common themes for the use of mobile health apps in primary breast cancer prevention: knowledge and adherence to screening guidelines, the targeting of high-risk populations, and the incorporation of theoretical frameworks. Primary prevention studies focused on apps that increased breast cancer prevention knowledge and adherence to breast cancer guidelines and surveillance (77, 79, 80, 83–85). Six of the 9 studies used existing guidelines to inform their apps (77, 80, 81, 83, 85). For example, in designing an app to help women reduce their risk of breast cancer through healthy behaviors, Coughlin et al. (81) included evidence-based information provided by the National Cancer Institute, the Centers for Disease Control and Prevention, and the American Cancer Society. In addition, a protocol study that provided healthy food recipes through the app aimed to assess adherence to diet and physical activity guidelines for cancer survivors set out by the American Institute for Cancer Research (85) and the investigators of an effectiveness study based the content of their app on the Saudi Cancer Foundation guidelines (77). Four studies focused on encouraging healthy behaviors that reduced the risk of breast cancer (78, 81, 82, 85).
The targeted population for these primary prevention studies was primarily women at high risk for breast cancer (77, 79, 80, 82, 83) including post-menopausal women with high Gail risk scores (82), BRCA mutation carriers (79, 80), and African American women, who experience greater breast cancer disparities (85). Some studies also targeted broader populations that engaged in behaviors associated with higher breast cancer risk, such as smoking (78) and night shift work (26). In the latter, Loef et al. described the protocol for an observational cohort of health workers in the Netherlands in which an app will be used to collect daily measures of infection to investigate how night shift work impacts health outcomes that are related to carcinogenesis (26). Therefore, apps are used both to increase knowledge about breast cancer risk and prevention in targeted populations (78, 85), as well as to identify new risk factors in high risk populations (26).
Many of the primary prevention studies incorporated theoretical frameworks for behavior change. The development studies incorporated the Common Sense Model of Behavior Theory (81), Health Information Model (83), and the Messaging Model for Health Communication Campaigns framework (80). One protocol study used both the Health Belief Theory and Theory of Planned Behavior Models (64). One effectiveness study based their study design on a Social Cognitive Theory (82). None of the feasibility studies mentioned a theoretical framework.
In addition to the three themes, we found that several key concepts were vital to implementing primary prevention research with apps, including literacy (specific to health and ehealth), self-efficacy (with a distinction between active and passive information seeking), and user-friendly scheduling tools. For example, literacy and self-efficacy were important in a study among college women that applied a family-based life course approach to breast cancer prevention (83). Given college-age women may adopt healthy lifestyles that are important for cancer risk reduction, Kratze et al. found that the app proved useful in knowledge transfer of breast health awareness while also assisting in daughter-initiated communication with their mothers regarding screenings and health information. The need for user-friendly tools, such as scheduling assistants, emerged in a study of guideline adherence among BRCA carriers. Although their awareness of surveillance guidelines was high, adherence was low and half of respondents indicated they had a difficult time remembering to schedule appointments (79). Thus, the app was designed to remind users when to seek care personalized to their own risk factors. The use of apps was particularly helpful in increasing effectiveness of behavioral interventions because they enabled dynamic tailoring in the case of smoking cessation (78) and easier self-monitoring in the case of tracking diet and physical activity (85).
With regard to app availability, 4 studies used publicly-available apps (Figure 5) (77, 79, 82, 84). Other studies used pre-existing apps, including My Fitness Pal (82), Snapchat (77), or incorporated their custom app to be used with FitBit and LoseIt! (81). The studies whose apps were not publicly-available either developed apps for research purposes only (85) or did not mention specific information about their app (26, 83). For one study, the author provided the app name upon contact (78).
Discussion
This systematic review summarizes the emerging literature for breast cancer prevention research using mobile apps. While we found studies across the cancer control continuum, the majority of studies used mobile apps to target tertiary prevention, particularly clinical care coordination and health-related quality of life for breast cancer survivors, as well as to improve the measurement and assessment of symptoms, behaviors, and risk. Fewer mobile apps were used for secondary and primary prevention where outcomes were related to increasing self-efficacy and screening behaviors and tracking and managing health behaviors. The studies reviewed spanned all phases of research in diverse populations in nearly 20 countries. The use of apps in breast cancer research has been increasing since 2010, a trend that will likely continue. Given the ubiquity of smartphones and global burden of breast cancer, there is potential for mobile apps to impact breast cancer trends across the globe.
Progress Since Previous Reviews
Previous reviews have explored the use of cancer apps, but were not systematically conducted (86), specific to breast cancer (87), or focused on research (4). That being said, our findings suggest that some of the gaps identified by past reviews have begun to be addressed. In particular, we identified that many of the primary prevention studies were grounded in theoretical frameworks and were tailored to different cultural and literacy levels, key points that were not being addressed previously as identified by Coughlin et al. (86). Similar to Coughlin et al. (86) and Giunti et al. (4), we also found that the majority of breast cancer apps were designed for tertiary prevention. We further observed that in studies of secondary and primary prevention, many apps provide information about guidelines for early detection of breast cancer for women identified as high risk. However, given that early onset breast cancer is increasing even in women without a family history of breast cancer, larger scale prevention interventions should be considered for additional populations that current risk models and screening strategies do not identify. We also found that apps could be adapted for studies across the cancer control continuum given that healthy behaviors recommended for primary and tertiary prevention overlap. Thus, in this rapidly growing field, while some gaps have been addressed, others gaps and implementation opportunities are emerging.
Research Gaps by Cancer Prevention Types
Tertiary Prevention Gaps
Given that breast cancer is the most commonly diagnosed cancer in women globally (88) and there are an estimated 3.5 million breast cancer survivors in the US alone (89), it makes sense that the majority of the apps were focused on clinical care coordination and health related quality of life. The majority of the apps we identified for tertiary breast cancer prevention were patient- or survivor-oriented; therefore, they required adherence from the patient/survivor. While this could place a considerable burden on patients/survivors, the repeat and real-time evidence gleaned can be invaluable for patients/survivors in terms of self-management. Furthermore, a small proportion (16%) of studies using apps for tertiary cancer prevention were effectiveness studies. Given the rising rates of breast cancer incidence in low-middle income countries (90), more studies are needed to show the effectiveness of app use, especially in low resource settings.
Secondary Prevention Gaps
While a greater proportion of secondary prevention studies were at the effectiveness stage, we found mixed evidence that apps could modify breast cancer screening behaviors, especially among at-risk populations. Lee et al. showed that a mobile phone-app based intervention, in combination with health navigator services, could effectively improve breast cancer knowledge and readiness for mammography (75). Ginsberg et al. also explored the effectiveness of an app, with or without a health navigator service, to increase Bangladeshi women's adherence to attend a clinic-visit after an abnormal clinical breast examination; however, no significant results were found (74). Similarly, an app in conjunction with genetic clinical counseling did not change women's personal perception of risk (18). Effectiveness studies ought to assess if an app could deliver substantial gains in secondary breast cancer prevention outcomes (e.g., education, screening), alone or in combination with other services. Moreover, given early detection of breast cancer is associated with greater survival rates, effectiveness studies that assess outcomes for the implementation of innovative breast cancer screening/detection apps compared to standard of care, would be of great value. This is especially true for areas where there are barriers to mammography screening and/or timely point-of-care diagnostics.
Primary Prevention Gaps
The majority of primary prevention studies were aimed at improving the transfer of knowledge and adherence to existing cancer prevention guidelines among women at high risk for breast cancer; however, less research has been conducted with populations at average risk, or on modifiable risk factors to prevent breast cancer. Targeted prevention to high-risk populations is logical given that with limited resources and competing disease risk, resources should be allocated to those who will benefit most. However, if maintaining healthy weight, diet and physical activity can reduce cancer incidence by 26% (91), then apps can help promote sustainable, scalable behavioral change that reduces the risk for many additional chronic diseases (e.g., heart disease, diabetes) for women at average risk as well.
Global Implementation Implications
As of early 2019, there were over 5.1 billion mobile phone subscribers and this number is growing given the average annual percent increase of 2.9% (92). One could argue that the adoption of smartphone use is faster than the rate of an epidemic. With smartphones, individuals are readily, in real time, self-monitoring health behaviors. And leveraging this self-tracking for the implementation of breast cancer prevention is at our fingertips. Our review suggests that the use of apps for breast cancer prevention is far-reaching. The global rise in incidence rates of breast cancer coupled with a rapid uptake of mobile platforms creates unique prevention opportunities. That being said, it is unclear if the use of apps for breast cancer prevention will mitigate or create greater gaps in health disparities (93). While low to middle income countries have experienced rapid uptake of mobile platforms (94), in these emerging markets, the young, well-educated and higher-income individuals are more likely to use these mobile platforms (93). Thus, an unintended consequence is the creation of breast cancer health disparities in low resource settings; especially for secondary and tertiary prevention. But, thoughtful app developments and implementation of mHealth tools could lead to more inclusive rather than marginalized research (93).
Opportunities and Recommendations of Mobile App Use Across the Cancer Control Continuum
Given our review, we highlight the following opportunities and/or recommendations with regard to the use of apps across the breast cancer control continuum:
Research is needed to understand the effectiveness of mobile apps for breast cancer primary prevention in women at average risk, but especially in young women. The incidence of invasive breast cancer in young women (age 25–39 years) has risen in the US with an annual percent change of 2.7% for white non-Hispanic women and 3.1% for black non-Hispanic women from 1976 to 2009 (1). Moreover, while global incidence rates for young women under 50 years are similar, independent of country-level income, mortality rates are higher in women in low-middle income and low-income countries (95). Many behavioral risk factors for breast cancer are modifiable, so the potential impact of app technology for breast cancer prevention in young women is particularly powerful given that this age group has come of age with apps and they do not need to be taught or convinced of their usefulness (93).
Breast cancer apps should be readily available. Only about half of the apps in our review were publicly available in the Apple and/or Android app store. The majority of apps readily available for public use were health related apps; whereas, apps catering to secondary prevention (breast cancer screening/detection) and tertiary prevention (continuing cancer care) were not readily available. Even for primary prevention, Cohen et al. found that over 200 potential users from 68 countries outside of the US tried to access the SNAP for BRCA app, but potential users could not download the app as it required a study code (79). Without making developed apps readily available and usable, there is limited possibility of updating, adapting, validating, disseminating, or further testing the app for effectiveness in diverse populations and settings. Researchers should also take advantage of already available apps, especially popular ones (e.g., Fitbit, Headspace), as there is less upfront person time and financial expenses compared to de novo app development. Popular apps carry the benefit of having a strong infrastructure given that software is routinely updated, designs are improved, and new features are added (82). However, an inherent limitation of readily available apps is that the speed of the research does not often advance at the speed of mobile app technology; therefore, researchers have limited control over app developments and the changes that may directly or indirectly impact the study.
Researchers should capitalize on the opportunity apps provide to collect information on exposures and outcomes of interest that have traditionally been difficult to measure. Not only does mobile app technology allow researchers to obtain repeat real-time data, mobile data measurement and collection reduces in-person study staff assistance, while not fully replacing study staff. Study staff will likely remain essential, especially for study implementation in low-middle income and hard to reach populations (84).
Limitations
This review is not without limitations. First, the advent of mobile apps is relatively recent and research in this area is rapidly changing. As a result, articles may have been missed that were not indexed with the search terms selected. To counteract this possibility, we broadened our search to include the full-text rather than just MeSH or keywords. Second, our review may also be missing studies that addressed breast cancer risk factors, such as obesity, but do not make an explicit reference to breast cancer. This likely deflated the number of articles identified as primary prevention; however, a more exhaustive review of all mobile apps being used for breast cancer risk factors was beyond the scope of this study. Finally, we included two databases in our search strategy, so gray literature and clinical trials with unpublished findings were not included.
Conclusions
The use of mobile apps for breast cancer prevention research is rapidly growing. Our systematic review suggests that while some gaps identified in previous reviews have already been addressed, new challenges have emerged. For mobile app interventions to have a global impact across the cancer control continuum, researchers will need to continue to invest in primary and secondary prevention research studies, as well as studies that are farther along in the research phase, in order to demonstrate the potential impact on outcomes relevant to breast cancer.
Author Contributions
LH and JM conceptualized the study and all authors (LH, RH, JM) formulated the study design. RH managed the literature search and reviewed all articles and LH and JM independently reviewed a subset of articles. All authors drafted the initial manuscript, reviewed and revised the final manuscript for critical and important intellectual content, approved the final manuscript, and agree to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the organizers of the 8th International Breast Cancer and Nutrition Symposium for the excellent feedback that encouraged this review during our session on mobilizing breast cancer research through smartphone apps. We would also like to thank Ms. Eisha Nasar for generating the map included in this review.
Footnotes
Funding. The National Cancer Institute supported both JM (5 K01 CA186943) and LH (5 K07 CA218166).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2019.00298/full#supplementary-material
References
- 1.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. (2013) 309:800–5. 10.1001/jama.2013.776 [DOI] [PubMed] [Google Scholar]
- 2.Sanson-Fisher RW, D'Este CA, Carey ML, Noble N, Paul CL. Evaluation of systems-oriented public health interventions: alternative research designs. Annu Rev Public Health. (2014) 35:9–27. 10.1146/annurev-publhealth-032013-182445 [DOI] [PubMed] [Google Scholar]
- 3.Bender JL, Yue RYK, To MJ, Deacken L, Jadad AR. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res. (2013) 15:e287 10.2196/jmir.2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giunti G, Giunta DH, Guisado-Fernandez E, Bender JL, Fernandez-Luque L. A biopsy of breast cancer mobile applications: state of the practice review. Int J Med Inform. (2018) 110:1–9. 10.1016/j.ijmedinf.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orsmond GI, Cohn ES. The distinctive features of a feasibility study: objectives and guiding questions. OTJR (Thorofare NJ). (2015) 35:169–77. 10.1177/1539449215578649 [DOI] [PubMed] [Google Scholar]
- 7.Hwang H. Electronic wound monitoring after ambulatory breast cancer surgery: improving patient care and satisfaction using a smart phone app. Br Col Med J. (2016) 58:448–53. [Google Scholar]
- 8.Armstrong KA, Coyte PC, Brown M, Beber B, Semple JL. Effect of home monitoring via mobile app on the number of in-person visits following ambulatory surgery a randomized clinical trial. JAMA Surg. (2017) 152:622–7. 10.1001/jamasurg.2017.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritvo P, Obadia M, Santa Mina D, Alibhai S, Sabiston C, Oh P, et al. Smartphone-enabled health coaching intervention (iMOVE) to promote long-term maintenance of physical activity in breast cancer survivors: protocol for a feasibility pilot randomized controlled trial. JMIR Res Protoc. (2017) 6:e165. 10.2196/resprot.6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiao L, Xu Z, Du W, Li H, Yin M. Fast preparation of polydopamine nanoparticles catalyzed by Fe(2+)/H2O2 for visible sensitive smartphone-enabled cytosensing. ACS Appl Mater Interfaces. (2017) 9:28339–45. 10.1021/acsami.7b10564 [DOI] [PubMed] [Google Scholar]
- 11.Xiaosheng D, Xiangren Y, Shuyuan H, Dezong G, Mengyao C, Meng D. The effects of combined exercise intervention based on Internet and social media software for postoperative patients with breast cancer: study protocol for a randomized controlled trial. Trials. (2018) 19:477. 10.1186/s13063-018-2857-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu JM, Ebert L, Liu XY, Wei D, Chan SWC. Mobile breast cancer e-support program for chinese women with breast cancer undergoing chemotherapy (part 2): multicenter randomized controlled trial. JMIR Mhealth Uhealth. (2018) 6:e104. 10.2196/mhealth.9438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drewes C, Kirkovits T, Schiltz D, Schinkoethe T, Haidinger R, Goldmann-Posch U, et al. EHealth acceptance and new media preferences for therapy assistance among breast cancer patients. JMIR Cancer. (2016) 2:e13. 10.2196/cancer.5711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egbring M, Far E, Roos M, Dietrich M, Brauchbar M, Kullak-Ublick GA, et al. A mobile app to stabilize daily functional activity of breast cancer patients in collaboration with the physician: a randomized controlled clinical trial. J Med Internet Res. (2016) 18:e238. 10.2196/jmir.6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Shafie RA, Weber D, Bougatf N, Sprave T, Oetzel D, Huber PE, et al. Supportive care in radiotherapy based on a mobile app: prospective multicenter survey. JMIR Mhealth Uhealth. (2018) 6:e10916. 10.2196/10916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien C, Kelly J, Lehane EA, Livingstone V, Cotter B, Butt A, et al. Validation and assessment of a technology familiarity score in patients attending a symptomatic breast clinic. World J Surg. (2015) 39:2441–9. 10.1007/s00268-015-3134-1 [DOI] [PubMed] [Google Scholar]
- 17.Foley NM, O'Connell EP, Lehane EA, Livingstone V, Maher B, Kaimkhani S, et al. PATI: Patient accessed tailored information: a pilot study to evaluate the effect on preoperative breast cancer patients of information delivered via a mobile application. Breast. (2016) 30:54–8. 10.1016/j.breast.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 18.Keohane D, Lehane E, Rutherford E, Livingstone V, Kelly L, Kaimkhani S, et al. Can an educational application increase risk perception accuracy amongst patients attending a high-risk breast cancer clinic? Breast. (2017) 32:192–8. 10.1016/j.breast.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 19.Heo J, Chun M, Lee KY, Oh YT, Noh OK, Park RW. Effects of a smartphone application on breast self-examination: a feasibility study. Healthc Inform Res. (2013) 19:250–60. 10.4258/hir.2013.19.4.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min YH, Lee JW, Shin YW, Jo MW, Sohn G, Lee JH, et al. Daily collection of self-reporting sleep disturbance data via a smartphone app in breast cancer patients receiving chemotherapy: a feasibility study. J Med Internet Res. (2014) 16:e135. 10.2196/jmir.3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Lim S, Min YH, Shin YW, Lee B, Sohn G, et al. Depression screening using daily mental-health ratings from a smartphone application for breast cancer patients. J Med Internet Res. (2016) 18:e216. 10.2196/jmir.5598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uhm KE, Yoo JS, Chung SH, Lee JD, Lee I, Kim JI, et al. Effects of exercise intervention in breast cancer patients: is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res Treat. (2017) 161:443–52. 10.1007/s10549-016-4065-8 [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Kim SM, Shin H, Jang JS, Kim YI, Han DH. A mobile game for patients with breast cancer for chemotherapy self-management and quality-of-life improvement: randomized controlled trial. J Med Internet Res. (2018) 20:e273. 10.2196/jmir.9559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SW, Lee I, Kim JI, Park H, Lee JD, Uhm KE, et al. Factors associated with physical activity of breast cancer patients participating in exercise intervention. Support Care Cancer. (2019) 27:1747–54. 10.1007/s00520-018-4427-3 [DOI] [PubMed] [Google Scholar]
- 25.Timmerman JG, Dekker-van Weering MGH, Tonis TM, Hermens HJ, Vollenbroek-Hutten MMR. Relationship between patterns of daily physical activity and fatigue in cancer survivors. Eur J Oncol Nurs. (2015) 19:162–8. 10.1016/j.ejon.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 26.Loef B, van Baarle D, van der Beek AJ, van Kerkhof LW, van de Langenberg D, Proper KI. Klokwerk plus study protocol: an observational study to the effects of night-shift work on body weight and infection susceptibility and the mechanisms underlying these health effects. BMC Public Health. (2016) 16:692 10.1186/s12889-016-3317-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young-Afat DA, van Gils CH, Bruinvels DJ, van der Pol CC, Witkamp AJ, Sijtsema S, et al. Patients' and health care providers' opinions on a supportive health app during breast cancer treatment: a qualitative evaluation. JMIR Cancer. (2016) 2:e8. 10.2196/cancer.5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douma JAJ, Verheul HMW, Buffart LM. Feasibility, validity and reliability of objective smartphone measurements of physical activity and fitness in patients with cancer. BMC Cancer. (2018) 18:1052. 10.1186/s12885-018-4983-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ormel HL, van der Schoot GGF, Westerink NDL, Sluiter WJ, Gietema JA, Walenkamp AME. Self-monitoring physical activity with a smartphone application in cancer patients: a randomized feasibility study (SMART-trial). Support Care Cancer. (2018) 26:3915–23. 10.1007/s00520-018-4263-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lozano-Lozano M, Martin-Martin L, Galiano-Castillo N, Alvarez-Salvago F, Cantarero-Villanueva I, Fernandez-Lao C, et al. Integral strategy to supportive care in breast cancer survivors through occupational therapy and a m-health system: design of a randomized clinical trial. BMC Med Inform Decis Mak. (2016) 16:150. 10.1186/s12911-016-0394-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paredes-Aracil E, Palazon-Bru A, Folgado-de la Rosa DM, Ots-Gutierrez JR, Compan-Rosique AF, Gil-Guillen VF. A scoring system to predict breast cancer mortality at 5 and 10 years. Sci Rep. (2017) 7:415. 10.1038/s41598-017-00536-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weaver A, Love SB, Larsen M, Shanyinde M, Waters R, Grainger L, et al. A pilot study: dose adaptation of capecitabine using mobile phone toxicity monitoring–supporting patients in their homes. Support Care Cancer. (2014) 22:2677–85. 10.1007/s00520-014-2224-1 [DOI] [PubMed] [Google Scholar]
- 33.Harder H, Holroyd P, Burkinshaw L, Watten P, Zammit C, Harris PR, et al. A user-centred approach to developing bWell, a mobile app for arm and shoulder exercises after breast cancer treatment. J Cancer Surviv. (2017) 11:732–42. 10.1007/s11764-017-0630-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brett J, Boulton M, Watson E. Development of an e-health app to support women prescribed adjuvant endocrine therapy after treatment for breast cancer. Patient Prefer Adher. (2018) 12:2639–47. 10.2147/ppa.s187692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts AL, Potts HWW, Koutoukidis DA, Smith L, Fisher A. Breast, prostate, and colorectal cancer survivors' experiences of using publicly available physical activity mobile apps: qualitative study. JMIR Mhealth Uhealth. (2019) 7:e10918. 10.2196/10918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ainsworth MC, Pekmezi D, Bowles H, Ehlers D, McAuley E, Courneya KS, et al. Acceptability of a mobile phone app for measuring time use in breast cancer survivors (life in a day): mixed-methods study. JMIR Cancer. (2018) 4:e9. 10.2196/cancer.8951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akechi T, Yamaguchi T, Uchida M, Imai F, Momino K, Katsuki F, et al. Smartphone problem-solving and behavioural activation therapy to reduce fear of recurrence among patients with breast cancer (SMartphone Intervention to LEssen fear of cancer recurrence: SMILE project): protocol for a randomised controlled trial. BMJ Open. (2018) 8:e024794. 10.1136/bmjopen-2018-024794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali EE, Leow JL, Chew L, Yap KY. Patients' perception of app-based educational and behavioural interventions for enhancing oral anticancer medication adherence. J Cancer Educ. (2018) 33:1306–13. 10.1007/s13187-017-1248-x [DOI] [PubMed] [Google Scholar]
- 39.Armstrong KA, Coyte PC, Bhatia RS, Semple JL. The effect of mobile app home monitoring on number of in-person visits following ambulatory surgery: protocol for a randomized controlled trial. JMIR Res Protoc. (2015) 4:e65. 10.2196/resprot.4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banas JR, Victorson D, Gutierrez S, Cordero E, Guitleman J, Haas N. Developing a peer-to-peer mHealth application to connect hispanic cancer patients. J Cancer Educ. (2017) 32:158–65. 10.1007/s13187-016-1066-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baseman J, Revere D, Baldwin LM. A mobile breast cancer survivorship care app: pilot study. JMIR Cancer. (2017) 3:e14. 10.2196/cancer.8192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buscemi J, Buitrago D, Iacobelli F, Penedo F, Maciel C, Guitleman J, et al. Feasibility of a smartphone-based pilot intervention for Hispanic breast cancer survivors: a brief report. Transl Behav Med. (2019) 9:638–45. 10.1093/tbm/iby058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iacobelli F, Adler RF, Buitrago D, Buscemi J, Corden ME, Perez-Tamayo A, et al. Designing an mHealth application to bridge health disparities in Latina breast cancer survivors: a community-supported design approach. Design Health (Abingdon). (2018) 2:58–76. 10.1080/24735132.2018.1452871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanez BR, Buitrago D, Buscemi J, Iacobelli F, Adler RF, Corden ME, et al. Study design and protocol for My Guide: an e-health intervention to improve patient-centered outcomes among Hispanic breast cancer survivors. Contemp Clin Trials. (2018) 65:61–8. 10.1016/j.cct.2017.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chalela P, Munoz E, Inupakutika D, Kaghyan S, Akopian D, Kaklamani V, et al. Improving adherence to endocrine hormonal therapy among breast cancer patients: Study protocol for a randomized controlled trial. Contemp Clin Trials Commun. (2018) 12:109–15. 10.1016/j.conctc.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delrieu L, Perol O, Fervers B, Friedenreich C, Vallance J, Febvey-Combes O, et al. A personalized physical activity program with activity trackers and a mobile phone app for patients with metastatic breast cancer: protocol for a single-arm feasibility trial. JMIR Res Protoc. (2018) 7:e10487. 10.2196/10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gehrke A, Lee SS, Hilton K, Ganster B, Trupp R, McCullough C, et al. Development of the cancer survivor profile-breast cancer (CSPro-BC) app: patient and nurse perspectives on a new navigation tool. J Cancer Surviv. (2018) 12:291–305. 10.1007/s11764-017-0668-2 [DOI] [PubMed] [Google Scholar]
- 48.Klasnja P, Hartzler A, Powell C, Pratt W. Supporting cancer patients' unanchored health information management with mobile technology. AMIA Annu Symp Proc. (2011) 2011:732–41. [PMC free article] [PubMed] [Google Scholar]
- 49.Klasnja P, Hartzler A, Powell C, Phan G, Pratt W. Health weaver mobile: designing a mobile tool for managing personal health information during cancer care. AMIA Annu Symp Proc. (2010) 2010:392–6. [PMC free article] [PubMed] [Google Scholar]
- 50.Kubo A, Altschuler A, Kurtovich E, Hendlish S, Laurent CA, Kolevska T, et al. A pilot mobile-based mindfulness intervention for cancer patients and their informal caregivers. Mindfulness. (2018) 9:1885–94. 10.1007/s12671-018-0931-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langer SL, Romano JM, Todd M, Strauman TJ, Keefe FJ, Syrjala KL, et al. Links between communication and relationship satisfaction among patients with cancer and their spouses: results of a fourteen-day smartphone-based ecological momentary assessment study. Front Psychol. (2018) 9:1843. 10.3389/fpsyg.2018.01843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langius-Eklof A, Crafoord MT, Christiansen M, Fjell M, Sundberg K. Effects of an interactive mHealth innovation for early detection of patient-reported symptom distress with focus on participatory care: protocol for a study based on prospective, randomised, controlled trials in patients with prostate and breast cancer. BMC Cancer. (2017) 17:466. 10.1186/s12885-017-3450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lloyd GR, Oza S, Kozey-Keadle S, Pellegrini CA, Conroy DE, Penedo FJ, et al. Breast cancer survivors' beliefs and preferences regarding technology-supported sedentary behavior reduction interventions. AIMS Public Health. (2016) 3:592–614. 10.3934/publichealth.2016.3.592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lozano-Lozano M, Galiano-Castillo N, Martin-Martin L, Pace-Bedetti N, Fernandez-Lao C, Arroyo-Morales M, et al. Monitoring energy balance in breast cancer survivors using a mobile app: reliability study. JMIR Mhealth Uhealth. (2018) 6:e67. 10.2196/mhealth.9669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lyons EJ, Baranowski T, Basen-Engquist KM, Lewis ZH, Swartil MC, Jennings K, et al. Testing the effects of narrative and play on physical activity among breast cancer survivors using mobile apps: study protocol for a randomized controlled trial. BMC Cancer. (2016) 16:202. 10.1186/s12885-016-2244-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCarroll ML, Armbruster S, Pohle-Krauza RJ, Lyzen AM, Min S, Nash DW, et al. Feasibility of a lifestyle intervention for overweight/obese endometrial and breast cancer survivors using an interactive mobile application. Gynecol Oncol. (2015) 137:508–15. 10.1016/j.ygyno.2014.12.025 [DOI] [PubMed] [Google Scholar]
- 57.Paredes-Aracil E, Palazon-Bru A, Folgado-de la Rosa DM, Ots-Gutierrez JR, Llorca-Ferrandiz C, Alonso-Hernandez S, et al. A scoring system to predict recurrence in breast cancer patients. Surg Oncol. (2018) 27:681–7. 10.1016/j.suronc.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 58.Lee HY, Lee MH, Gao Z, Sadak K. Development and evaluation of culturally and linguistically tailored mobile app to promote breast cancer screening. J Clin Med. (2018) 7:E181. 10.3390/jcm7080181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phillips SM, Collins LM, Penedo FJ, Courneya KS, Welch W, Cottrell A, et al. Optimization of a technology-supported physical activity intervention for breast cancer survivors: Fit2Thrive study protocol. Contemp Clin Trials. (2018) 66:9–19. 10.1016/j.cct.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pope Z, Lee JE, Zeng N, Lee HY, Gao Z. Feasibility of smartphone application and social media intervention on breast cancer survivors' health outcomes. Transl Behav Med. (2019) 9:11–22. 10.1093/tbm/iby002 [DOI] [PubMed] [Google Scholar]
- 61.Quintiliani LM, Mann DM, Puputti M, Quinn E, Bowen DJ. Pilot and feasibility test of a mobile health-supported behavioral counseling intervention for weight management among breast cancer survivors. JMIR Cancer. (2016) 2:e4. 10.2196/cancer.5305 [DOI] [PubMed] [Google Scholar]
- 62.Raghunathan NJ, Korenstein D, Li QS, Tonorezos ES, Mao JJ. Determinants of mobile technology use and smartphone application interest in cancer patients. Cancer Med. (2018) 7:5812–9. 10.1002/cam4.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosen KD, Paniagua SM, Kazanis W, Jones S, Potter JS. Quality of life among women diagnosed with breast cancer: a randomized waitlist controlled trial of commercially available mobile app-delivered mindfulness training. Psychooncology. (2018) 27:2023–30. 10.1002/pon.4764 [DOI] [PubMed] [Google Scholar]
- 64.Smith SA, Whitehead MS, Sheats JQ, Fontenot B, Alema-Mensah E, Ansa B. Formative research to develop a lifestyle application (app) for African American breast cancer survivors. J Ga Public Health Assoc. (2016) 6:50–9. 10.21633/jgpha.6.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soto-Perez-De-Celis E, Kim H, Rojo-Castillo MP, Sun CL, Chavarri-Guerra Y, Navarrete-Reyes AP, et al. A pilot study of an accelerometer-equipped smartphone to monitor older adults with cancer receiving chemotherapy in Mexico. J Geriatr Oncol. (2018) 9:145–51. 10.1016/j.jgo.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 66.Stubbins R, He T, Yu X, Puppala M, Ezeana CF, Chen S, et al. A behavior-modification, clinical-grade mobile application to improve breast cancer survivors' accountability and health outcomes. JCO Clin Cancer Inform. (2018) 2:1–11. 10.1200/cci.18.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valle CG, Deal AM, Tate DF. Preventing weight gain in African American breast cancer survivors using smart scales and activity trackers: a randomized controlled pilot study. J Cancer Surviv. (2017) 11:133–48. 10.1007/s11764-016-0571-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker DK, Hardeman A, Owen L, Frank JS. Information at the point of care an informational application for cancer resources. Cin Comput Inform Nurs. (2015) 33:390–5. 10.1097/cin.0000000000000171 [DOI] [PubMed] [Google Scholar]
- 69.Zhang X, Deng Z, Parvinzamir F, Dong F. MyHealthAvatar lifestyle management support for cancer patients. Ecancermedicalscience. (2018) 12:849. 10.3332/ecancer.2018.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu J, Ebert L, Guo D, Yang S, Han Q, Chan SW. Mobile breast cancer e-support program for Chinese women with breast cancer undergoing chemotherapy (part 1): qualitative study of women's perceptions. JMIR Mhealth Uhealth. (2018) 6:e85. 10.2196/mhealth.9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu J, Ebert L, Liu X, Chan SW. A mobile application of breast cancer e-support program versus routine care in the treatment of Chinese women with breast cancer undergoing chemotherapy: study protocol for a randomized controlled trial. BMC Cancer. (2017) 17:291. 10.1186/s12885-017-3276-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cardos RAI, Soflau R, Gherman A, Sucala M, Chiorean A. A mobile intervention for core needle biopsy related pain and anxiety: a usability study. J Evid Based Psychother. (2017) 17:21–30. 10.24193/jebp.2017.1.2 [DOI] [Google Scholar]
- 73.Eden KB, Scariati P, Klein K, Watson L, Remiker M, Hribar M, et al. Mammography decision aid reduces decisional conflict for women in their forties considering screening. J Womens Health (Larchmt). (2015) 24:1013–20. 10.1089/jwh.2015.5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ginsburg OM, Chowdhury M, Wu W, Chowdhury M, Pal BC, Hasan R, et al. An mHealth model to increase clinic attendance for breast symptoms in rural Bangladesh: can bridging the digital divide help close the cancer divide? Oncologist. (2014) 19:177–85. 10.1634/theoncologist.2013-0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee H, Ghebre R, Le C, Jang YJ, Sharratt M, Yee D. Mobile phone multilevel and multimedia messaging intervention for breast cancer screening: pilot randomized controlled trial. JMIR Mhealth Uhealth. (2017) 5:e154. 10.2196/mhealth.7091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tewary S, Arun I, Ahmed R, Chatterjee S, Chakraborty C. SmartIHC-Analyzer: smartphone assisted microscopic image analytics for automated Ki-67 quantification in breast cancer evaluation. Anal Methods. (2017) 9:6161–70. 10.1039/c7ay02302b [DOI] [Google Scholar]
- 77.Alanzi TM, Alobrah A, Alhumaidi R, Aloraifi S. Evaluation of the SnapChat mobile social networking application for breast cancer awareness among Saudi students in the Dammam Region of the Kingdom of Saudi Arabia. Breast Cancer. (2018) 10:113–9. 10.2147/bctt.s166135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Businelle MS, Ma P, Kendzor DE, Frank SG, Wetter DW, Vidrine DJ. Using intensive longitudinal data collected via mobile phone to detect imminent lapse in smokers undergoing a scheduled quit attempt. J Med Internet Res. (2016) 18:e275. 10.2196/jmir.6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cohen SA, Scherr CL, Nixon DM. An iPhone application intervention to promote surveillance among women with a BRCA mutation: pre-intervention data. J Genet Couns. (2018) 27:446–56. 10.1007/s10897-018-0224-x [DOI] [PubMed] [Google Scholar]
- 80.Scherr CL, Feuston JL, Nixon DM, Cohen SA. A two-phase approach to developing SNAP: an iPhone application to support appointment scheduling and management for women with a BRCA mutation. J Genet Couns. (2018) 27:439–45. 10.1007/s10897-018-0222-z [DOI] [PubMed] [Google Scholar]
- 81.Coughlin SS, Besenyi GM, Bowen D, De Leo G. Development of the Physical activity and Your Nutrition for Cancer (PYNC) smartphone app for preventing breast cancer in women. Mhealth. (2017) 3:5. 10.21037/mhealth.2017.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hartman SJ, Nelson SH, Cadmus-Bertram LA, Patterson RE, Parker BA, Pierce JP. Technology- and phone-based weight loss intervention: pilot RCT in women at elevated breast cancer risk. Am J Prev Med. (2016) 51:714–21. 10.1016/j.amepre.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kratzke C, Amatya A, Vilchis H. Differences among college women for breast cancer prevention acquired information-seeking, desired apps and texts, and daughter-initiated information to mothers. J Community Health. (2014) 39:291–300. 10.1007/s10900-013-9759-9 [DOI] [PubMed] [Google Scholar]
- 84.Bravo C, O'Donoghue C, Kaplan CP, Luce J, Ozanne E. Can mHealth improve risk assessment in underserved populations? Acceptability of a breast health questionnaire app in ethnically diverse, older, low-income women. J Health Dispar Res Pract. (2014) 7:6. [PMC free article] [PubMed] [Google Scholar]
- 85.Smith SA, Whitehead MS, Sheats J, Mastromonico J, Yoo W, Coughlin SS. A community-engaged approach to developing a mobile cancer prevention app: the mCPA study protocol. JMIR Res Protoc. (2016) 5:e34. 10.2196/resprot.5290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coughlin SS, Thind H, Liu B, Wilson LC. Towards research-tested smartphone applications for preventing breast cancer. Mhealth. (2016) 2:26. 10.21037/mhealth.2016.06.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davis SW, Oakley-Girvan I. mHealth education applications along the cancer continuum. J Cancer Educ. (2015) 30:388–94. 10.1007/s13187-014-0761-4 [DOI] [PubMed] [Google Scholar]
- 88.World Health Organization Global Status Report on Noncommunicable Diseases 2014. Geneva: World Health Organization; (2014). [Google Scholar]
- 89.American Cancer Society Cancer Treatment & Survivorship: Facts & Figures 2016–2017. Atlanta, GA: American Cancer Society; (2016). [Google Scholar]
- 90.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. (2016) 25:16–27. 10.1158/1055-9965.epi-15-0578 [DOI] [PubMed] [Google Scholar]
- 91.Colditz GA, Bohlke K. Priorities for the primary prevention of breast cancer. CA Cancer J Clin. (2014) 64:186–94. 10.3322/caac.21225 [DOI] [PubMed] [Google Scholar]
- 92.The GSMA Corporate Website GSMA. (2019). Available online at: https://www.gsma.com/ (accessed August 27, 2019).
- 93.Taylor K, Silver L. Smartphone Ownership Is Growing Rapidly Around the World, but Not Always Equally. (2019). Available online at: https://www.pewresearch.org/global/2019/02/05/smartphone-ownership-is-growing-rapidly-around-the-world-but-not-always-equally/ (accessed February 28, 2019).
- 94.DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. (2015) 24:1495–506. 10.1158/1055-9965.epi-15-0535 [DOI] [PubMed] [Google Scholar]
- 95.Bellanger M, Zeinomar N, Tehranifar P, Terry MB. Are global breast cancer incidence and mortality patterns related to country-specific economic development and prevention strategies? J Glob Oncol. (2018) 4:1–16. 10.1200/jgo.17.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.