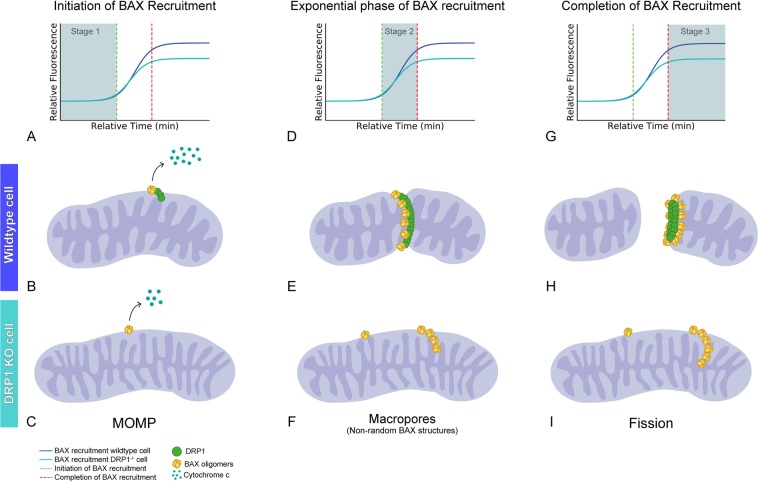
Figure 5.
Proposed model for BAX participation in mitochondrial fission during apoptosis. (A–C) Initiation of BAX recruitment (Stage 1) is comprised of BAX integration into the MOM, MOMP and cytochrome c release. At this stage, (A) the time to initiate BAX recruitment is indistinguishable between wild type and DRP1−/− cells. In wild type cells, DRP1 has already recruited to the MOM. (B,C) Cytochrome c is released in both cell types, however DRP1−/− cells show a reduction in the percentage of cells releasing cytochrome c. This difference may be related to a proposed function of DRP1 in facilitating transfer of cardiolipin, which is associated with cytochrome c, from the inner membrane to the MOM51. The release of other molecules, such as SMAC, is not inhibited in DRP1−/− cells48–50. BAX dimers and/or small oligomers are present at this stage. We define BAX structures at this stage as micropores. (D–F) The exponential phase of BAX recruitment (Stage 2) is defined as the period of BAX accumulation at the MOM. (D) The rate of BAX recruitment does not differ between wild type and DRP1−/− cells. (E) At the site of DRP1 constriction of the mitochondria in wild type cells, BAX accumulation takes on higher order non-random structures, however (F) these structures lack organized localization at the scission site in the absence of DRP1. We define these BAX structures as macropore oligomers, and may be required for the release of larger molecules and/or evulsions of the mitochondrial inner membrane. (G–I) Completion of BAX recruitment (Stage 3) is identified as the point where the (G) BAX recruitment curve plateaus. (H) Importantly, this turning point is accompanied by mitochondrial fragmentation in wild type cells. DRP1-mediated recruitment of BAX to the mitochondrial scission sites allows for non-random BAX structures or rings that are organized to completely encircle the mitochondrion. This likely weakens membrane integrity through presence of many adjacent macropores, creating a perforated membrane, and allowing constriction pressure created by DRP1 and its assemblies to complete the membrane separation. We define these BAX structures as fission oligomers. This model, where BAX perforates the membrane, is consistent with a model originally proposed by Martinou and Youle29. (I) Conversely, BAX structures on DRP1-deficient mitochondria are unable to organize around DRP1 identified scission sites, therefore cannot provide a perforated region to facilitate mitochondrial fragmentation. Loss or impairment of DRP1 function also results in fewer BAX puncta that contain approximately 25% less BAX protein. Therefore, DRP1-mediated fission oligomers may evolve both by coalescence of macropore oligomers and late-stage recruitment of inactive latent BAX monomers. For example, P168A mutant BAX cannot normally recruit to the MOM in apoptotic cells, unless in the presence of wild type BAX protein42. Kinetic studies indicate that this recruitment process occurs only after wild type BAX nears the plateau phase of its recruitment28, when we predict that fission oligomers are being formed.