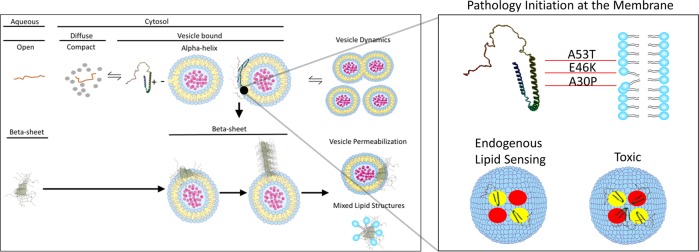
Fig. 2.
Potential role of lipids in αsyn aggregate pathoetiology. In the cell, αsyn is partitioned between aqueous phase and the lipid phase via transient interactions at the vesicle surface. Endogenous αsyn probably exists in several states, including a compact monomer and a vesicle-bound monomer with an N-terminal α-helix structure. Folding αsyn monomers on the vesicle surfaces likely plays a non-essential or redundant role in vesicle dynamics. β-Sheet confirmation of αsyn may begin at vesicle surfaces. Toxic effects of β-sheet oligomers included vesicle permeabilization or the formation of toxic mixed lipid–protein structures. Pathology initiation might involve specific configurations of αsyn folding onto a variety of membranes. Altered lipid-sensing properties by known disease-causing mutations (e.g., A30P, E46K, and A53T) might alter the affinity of αsyn for certain vesicle lipid components (depicted as yellow and red circles), or change the spatial arrangement of αsyn molecules on the vesicle surface. Resulting β-sheet oligomers may have different toxic or prion-like properties based the physiochemical details of the initial pathology development