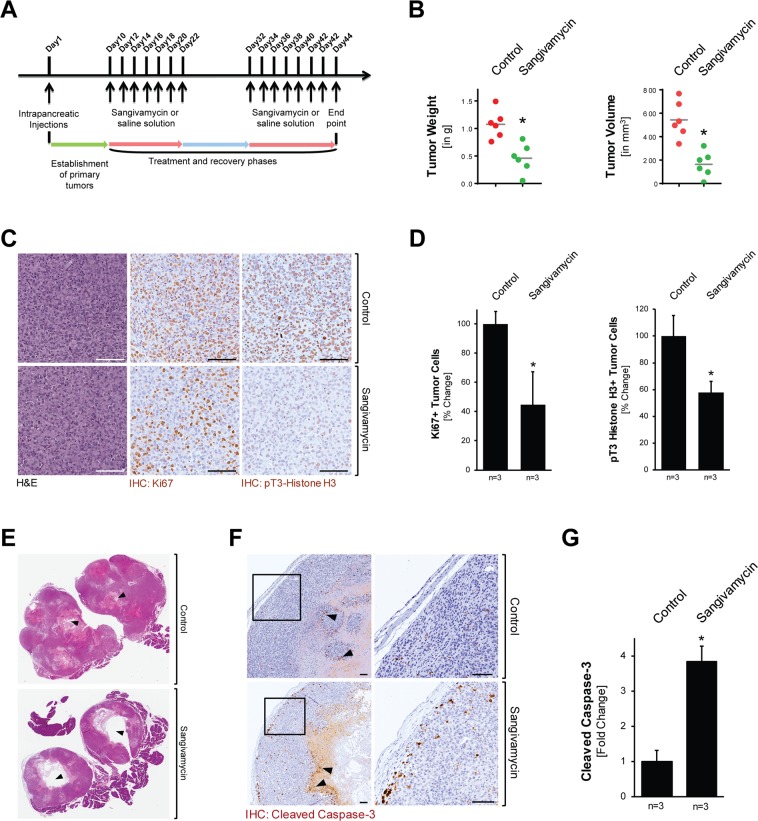
Figure 5.
Sangivamycin targets orthotopic PDA tumors in vivo. (A) Scheme of the timeline of the animal experiment to test the effects of Sangivamycin on orthotopic pancreatic tumors. (B) Analyses of tumor weight and tumor volume of mice (n = 6) after indicated treatment. The asterisk indicates statistical significance (p = 0.002 for tumor weight; p = 0.0006 for tumor volume). (C) Formalin-fixed tumor samples from mice treated with vehicle or Sangivamycin were analyzed by IHC for expression of either Ki67 or pT3-Histone H3. Shown are representative pictures. The bar represents 100 µm. (D) Quantification of IHC staining (n = 3 samples per treatment group; randomly selected) for tumor cells expressing Ki67 and phosphorylated Histone H3. The asterisk indicates statistical significance with p < 0.05. (E) H&E staining of tumors shows larger necrosis area (black arrow heads) in Sangivamycin-treated mice, as compared to vehicle-treated mice. (F) IHC analysis of control and treated mouse tumors (n = 3 per treatment group) using an anti-cleaved caspase-3 antibody. The bar represents 100 µm. (G) Quantification of IHC staining (n = 3 samples per treatment group) for apoptotic tumor cells (cleaved caspase-3 positive tumor cells). The asterisk indicates statistical significance with p < 0.05.