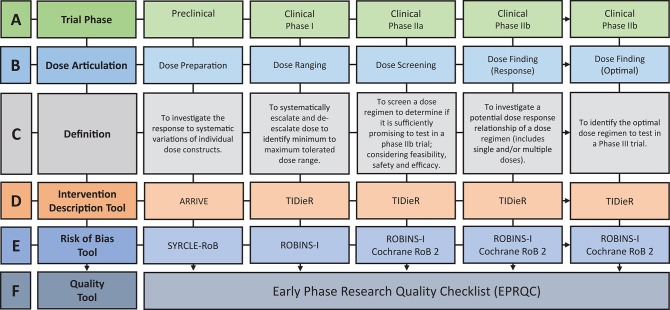
Figure 1.
Overview of the discovery pipeline. (A) Trial Phase: Preclinical: Studies involving animal subjects. Clinical: Studies involving human participants (12). (B) Dose articulation: Type of dose articulation trial relevant to phase. Example studies for each phase: Preclinical—Dose Preparation (13, 14). Clinical—Dose Ranging (15, 16), Clinical—Dose Screening (17) Clinical—Dose Finding (Response) (18, 19), Clinical—Dose Finding (Optimal) (20). (C) Definition: Definition of how dose is articulated in each phase. (D) Intervention Description Tool: ARRIVE: Animal Research: Reporting of in vivo Experiments (21). TIDieR: Template for Intervention Description and Replication Checklist (22). (E) Risk of Bias Tool: SYRCLE-RoB: Systematic Review Center for Laboratory Animal Experimentation's Risk of Bias Tool (23). Selection Bias: Is the population representative of population being analyzed. Cochrane RoB 2: Cochrane Risk of Bias 2 (24). ROBINS-I: Risk of Bias in Non-Randomized Studies-of Interventions (25). (F) Quality Tool: EPRQC: Early Phase Research Quality Checklist adapted from quality assessment checklist for phase I cancer trials (26).