Figure 7.
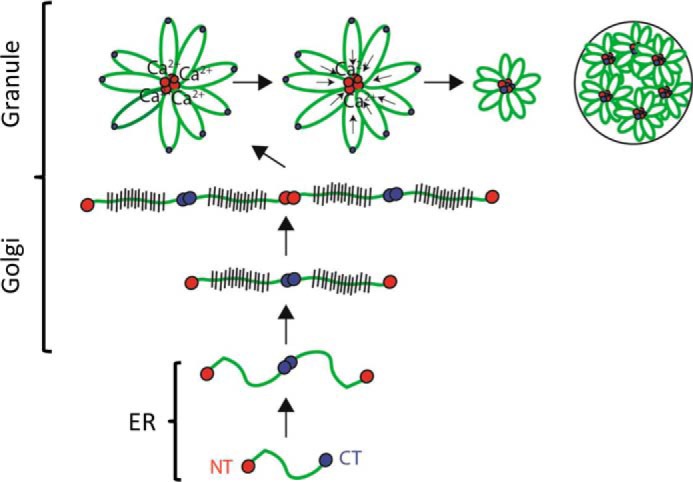
Model for MUC5B intracellular assembly and packaging. In the endoplasmic reticulum, MUC5B forms homotypic disulfide-linked dimers via its C-terminal dimerization domains (blue circles). The dimer is transported to the Golgi where it undergoes O-glycosylation prior to linear polymer formation via disulfide linkage between N-terminal multimerization domains (red circles). A reduction in pH and an increase in free calcium concentration occur across the secretory pathway. This results in noncovalent, calcium-mediated interactions between N termini, which appear as proteinaceous nodes, and aid organization of MUC5B for intragranular packaging. The new findings in this paper extend this model and establish that the C-terminal dimerization domains have the potential to interact with the multimerization domains at acidic pH in the presence of calcium, which may aid further compaction of the MUC5B molecule for intragranular packaging. The order of assembly of these N- and C-terminal containing nodes has yet to be elucidated. Uncoupling of these noncovalent interactions in mucus is critical for the transition to an expanded linear chains underpinning normal flowing mucus that can be transported by MCC to keep the lung free from infection. Addition of agents to mucus that displace calcium from mucins has been shown to normalize mucin conformation and decrease mucus viscoelasticity and improve MCC (64, 65).
