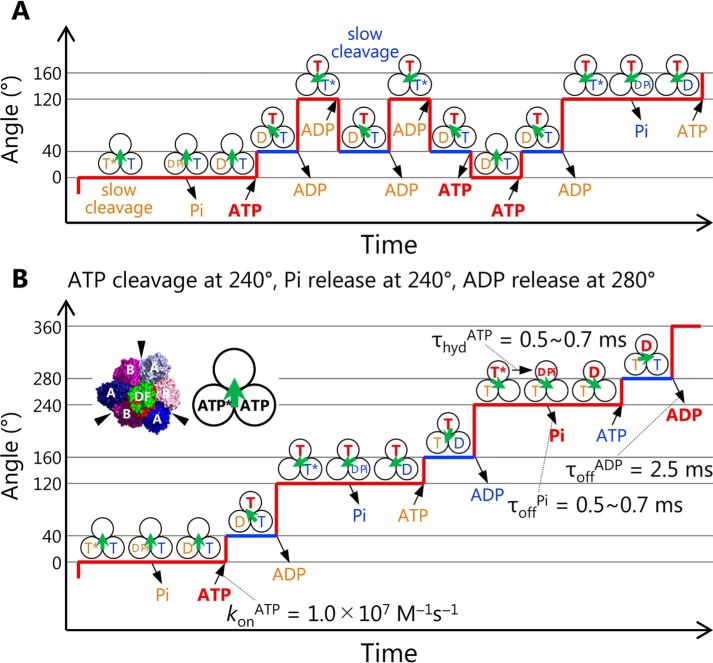
Figure 7.
A model of chemo-mechanical coupling of EhV1 including substeps and backward steps. A, model of backward steps described with the nucleotide states of three catalytic sites. Three black circles and a green arrow indicate the catalytic sites and the direction of rotor DF of EhV1, respectively. Under the conditions where bond cleavage durations are very long, such as ATPγS-driven rotation of WT or ATP-driven rotation of Arg-finger mutant, EhV1 shows backward steps in the presence of ADP. There are two kinds of the backward step, the −80° backward step from the main pause (red line) to the previous subpause (blue line) and the subsequent −40° backward step to the previous main pause. In our model, the −80 and −40° backward steps are triggered by ADP binding and ATP release, respectively. Also, the 40 and 80° recovery steps are triggered by ATP binding and ADP release, respectively. B, chemo-mechanical coupling scheme of EhV1 in normal rotation. During the main pause, ATP cleavage occurs, and Pi is released immediately. Then ATP binds to the empty site, and rotor DF rotates 40°. During the subpause, product ADP is released, and then the rotor DF subcomplex rotates 80°. In this scheme, when we focus on the single catalytic site on top, ATP (shown in red) binds at 0°, and then bound ATP is cleaved into ADP and Pi at 240°, Pi is released at the same angle, and ADP is released at 280°. In our model, the previous crystal structure corresponds to a state during the main pause.