Figure 1.
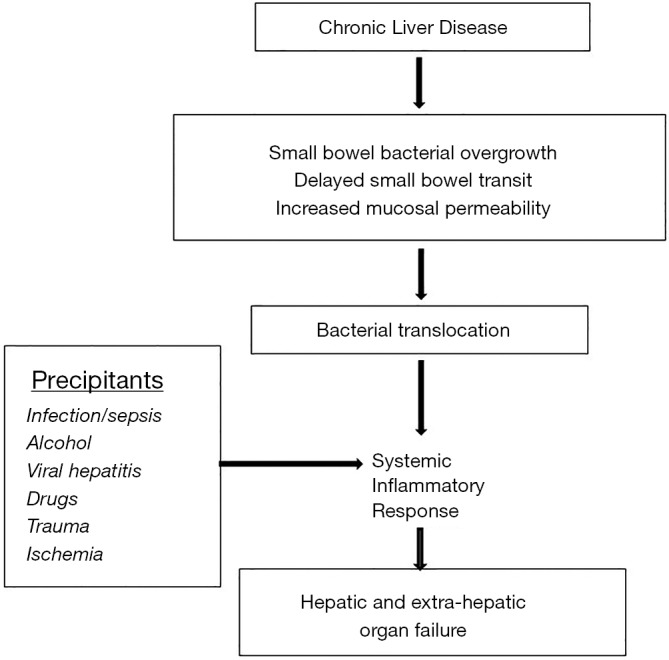
Pathogenesis of acute and chronic liver failure. Hepatic and systemic inflammation is hallmark of this syndrome. Interaction between the gastrointestinal tract and liver (gut-liver axis) plays a major role especially in patients with alcohol associated hepatitis. Changes in the gut with bacterial overgrowth and altered gut microbiome towards more harmful microbiome (dysbiosis) combined with increased intestinal permeability helps translocation of bacterial endotoxin and lipopolysaccharide, which results in activation of toll-like 4 receptors on the hepatic macrophages. Inflammatory pathways are activated via IL-1 family including tumor necrosis factor-alpha. Simultaneously, anti-inflammatory pathways get activated mediated by IL-10 family of cytokines. Severe ACLF with cytokine storm and hyperactive compensatory anti-inflammatory response (CARS) leads to immune paralysis setting the stage of worsening and progressive ACLF and development of bacterial or fungal infections. This is worsened in the presence of already immune compromised state in cirrhosis and use of corticosteroids in AAH. Dying and necrotic hepatocytes release cellular material with danger associate molecular patterns released perpetuating inflammation by activation of TLR-4 receptors on hepatic macrophages, and release extracellular vesicles also accentuating inflammation by recruitment of inflammatory cells and neutrophils especially in AAH. The inflamed hepatocytes also cross talk with vascular endothelial (ET) cells causing portal hypertension and with hepatic stellate cells (HSC) resulting their activation to lay down collagen tissue and development of fibrosis. ACLF, acute on chronic liver failure; AAH, alcohol associated hepatitis; TLR-4, activation of toll like-4.
