Figure 9.
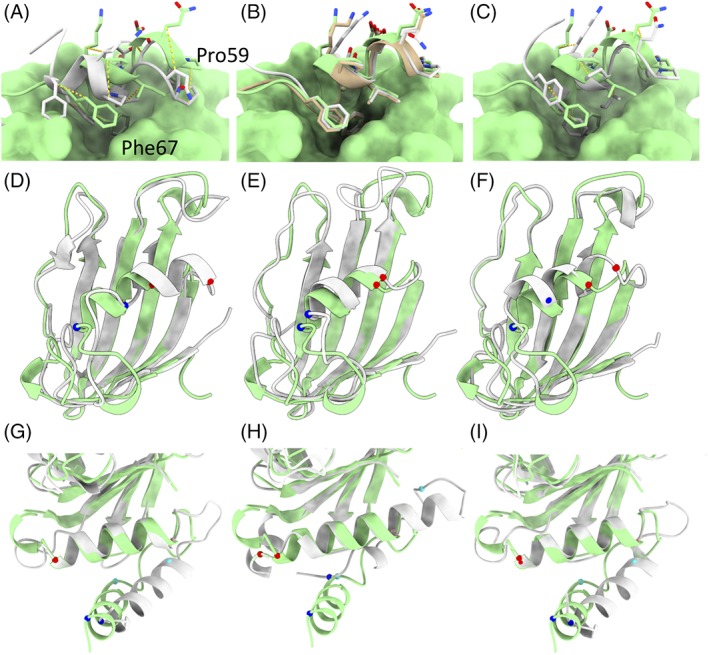
Comparison of Baker and Feig group results for three interesting cases. A‐C. Refinement target R0974s1 was a globular domain of five α‐helices, the first four of which were correct in the starting model. A. Starting model (gray) compared to target. The C‐terminal helix is tilted and shifted from its true position, with Ile62 packed into the core in place of Phe66. Equivalent Cβ atoms are connected by dashed yellow lines. The remainder of the target is shown in surface representation. B. The Baker (tan) and Feiglab (white) models matched the target essentially perfectly. C. The Baker‐Autorefine result improved upon the starting model, but did not quite reach the target conformation. D‐F. Refinement target R0981‐D4 was a particularly notable success for the Baker group. D. While the starting model (white) closely matched the main β‐sheet in the target, the helix spanning residues 434 (Cα shown in blue) to 441 (Cα in red) was shifted about 7.5 å from its true position. E. The Baker method shifted this helix to within 2 å of its true position, and correctly predicted the conformations of the entering and exiting turns. F. The next best result (from the Feig group) brought the helix to within 5 å of the target, but added a spurious extra turn to the N‐terminus. The first 17 residues of this domain were not correctly predicted by any group, and are not shown. G‐I. The N‐terminus of R0997, in contrast, highlights a potential pitfall of the use of fragment‐based sampling methods in refinement. G. In the starting model the first helix was essentially correctly folded, but turned almost 45° from its true configuration. Additionally, the somewhat large loops flanking the second helix were poorly modeled. H. the Baker group unfolded the N‐terminal helix, added two spurious extra turns to the N‐terminus of the second helix, and partially unwrapped the C‐terminal turn of the second helix in order to fold the following loop into a helix—a significant degradation of the model quality. On the other hand, the more conservative Feig method kept the secondary structure elements correctly folded and slightly improved the disposition of the N‐terminal helix and flanking loop geometry. Cα atoms equivalent to those constituting the N‐terminus and C‐terminus of the first two helices in the target are shown, colored in blue, cyan, pink, and red in order of residue number
