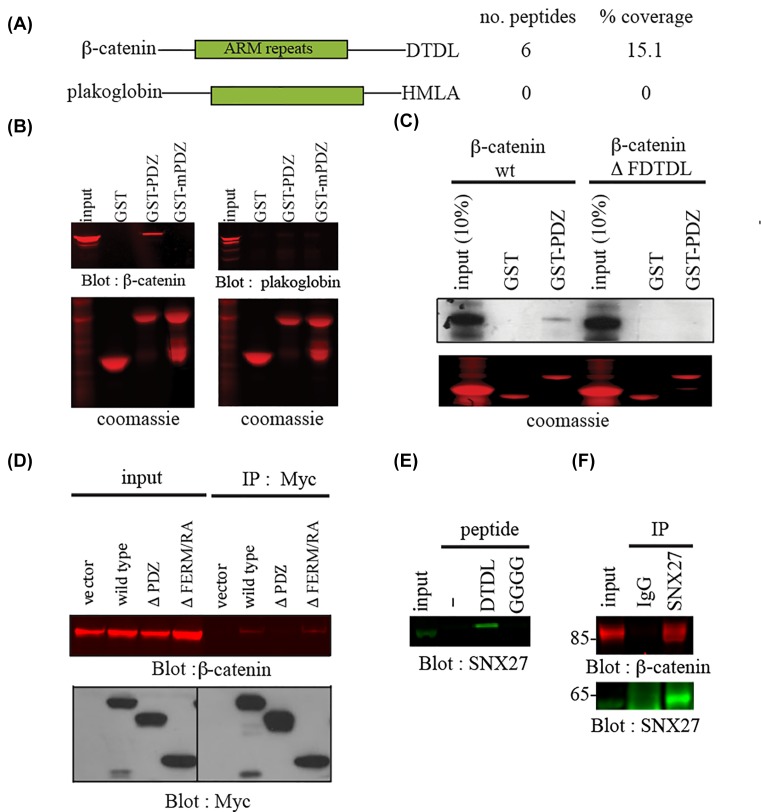
Figure 1. SNX27 binds β-catenin in mpkCCD cells.
(A) About 10 mg of mpkCCD cell lysate was incubated with a GST-fusion protein expressing the PDZ domain of SNX27. Interacting proteins were visualized by staining with Coomassie blue and identified by mass spectrometry as described in ‘Methods’ section. β-Catenin, but not closest relative plakoglobin, was detected in GST-PDZ bound complexes. (B) About 500 µg of mpkCCD was incubated with 10 µg GST or GST fusion proteins containing the SNX27 wild-type (GST-PDZ) or mutant (GST-mPDZ) PDZ domain known to abolish carboxy-terminal peptide ligand interactions. After washing, bound β-catenin or plakoglobin was detected by Western blotting. About 50 µg of lysates were loaded as control for Western blotting. Equal GST loading was verified by Coomassie staining. (C) About 1 µg of cDNAs of β-catenin wild-type or mutant lacking carboxy terminal PDZ-binding motif (ΔDTDL) were translated in the presence of [35S]-methionine. The translated product was incubated with GST or GST-fusion protein encoding the PDZ domain of SNX27 for 1 h at 4°C. The beads were washed, and bound translated product separated by SDS-PAGE. Detection of bound product was revealed by autoradiography. Twenty per cent of the translated product was used as input control. (D) MpkCCD cells were transiently transfected with empty Myc-tagged vector or plasmids containing Myc fusion proteins of the indicated SNX27 sequences. After 48 h incubation, the expressed fusion proteins were immunoprecipitated from cell lysates using a Myc antibody, and the immune complexes blotted for β-catenin (top) or Myc (bottom). (E) MpkCCD cell extracts were incubated with the indicated wild-type (DTDL) or mutant (GGGG) carboxy-terminal β-catenin peptides conjugated to streptavadin-agarose beads. Bound SNX27 was detected by Western blotting. (F) mpkCCD cell lysates (2 mg) were incubated with a SNX27 polyclonal antibody or preimmune serum (5 µl). Immune complexes were collected using protein A-Sepharose. Bound β-catenin was detected by Western blotting. About 50 µg of cell lysate was run as input control.