Figure 3.
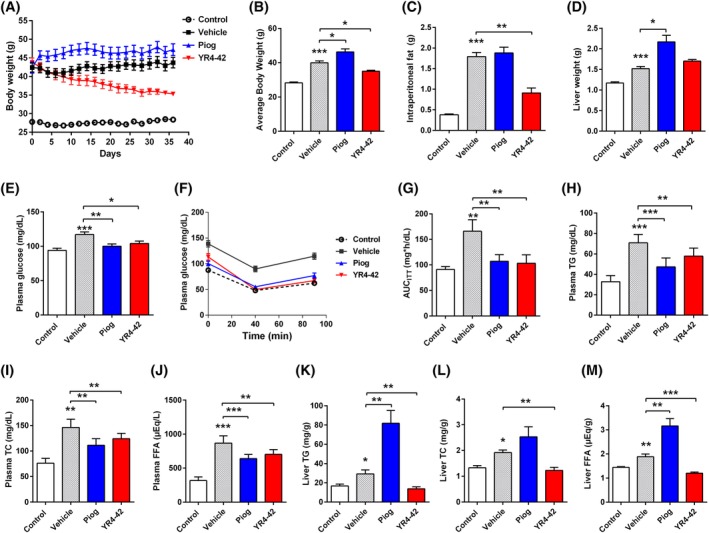
YR4‐42 ameliorates hyperglycaemia, hyperlipidaemia and hepatic steatosis of diet‐induced obese (DIO) mice without weight gain. DIO‐C57BL/6J mice were divided into three groups: Vehicle group (oral gavage of 0.5%CMC‐Na); Piog group (oral gavage of pioglitazone, 25 mg/kg) and YR4‐42 group (oral gavage of YR4‐42, 50 mg/kg). C57BL/6J mice fed with standard chow and oral gavage of 0.5%CMC‐Na represented the control group. A, Body weights of mice determined continuously during 38 days of oral administration. B, Average body weight of mice after 38 days of oral administration. C, D, Intraperitoneal fat C, and liver D of mice were weighted at the end of oral administration. E, Fasting blood glucose levels, determined on the 7th day. F, Insulin tolerance test, performed on the 14th day. G, Area under the curve of insulin tolerance test (AUCITT)measurements on the 14th day. H, Plasma triglyceride (TG), I, total cholesterol (TC), J, free fatty acid (FFA) levels in the mice, determined at the end of oral administration (on the 38th day). K, Liver TG, L, TC and M, FFA levels in the mice, determined at the end of oral administration (on the 38th day). All experiments were performed independently. Data are shown as mean ± SEM of every group, n = 12. *P < 0.05, **P < 0.01, ***P < 0.001, vs control group or the indicated group
