Abstract
Background
Hypothermia, either therapeutically induced or accidental (ie, an involuntary decrease in core body temperature to <35°C), results in hemostatic disorders. However, it remains unclear whether hypothermia enhances or inhibits coagulation, especially in severe hypothermia. The present study evaluated the thrombocytic and hemostatic changes in hypothermic mice.
Methods
C57Bl/6 mice were placed at an ambient temperature of −20°C under general anesthesia. When the rectal temperature decreased to 15°C, 10 mice were immediately euthanized, while another 10 mice were rewarmed, kept in normal conditions for 24 hours, and then euthanized. These treatments were also performed in 20 splenectomized mice.
Results
The hypothermic mice had adhesion of CD62P‐positive platelets with high expression of von Willebrand factor (vWF) in their spleens, while the status of the peripheral platelets was unchanged. Furthermore, the plasma levels of platelet factor 4 (PF4) and pro‐platelet basic protein (PPBP), which are biomarkers for platelet degranulation, were significantly higher in hypothermic mice than in control mice, indicating that hypothermia activated the platelets in the splenic pool. Thus, we analyzed these biomarkers in asplenic mice. There was no increase in either PF4 or PPBP in splenectomized hypothermic mice. Additionally, the plasma D‐dimer elevation and microthrombosis were caused in rewarmed mice, but not in asplenic rewarmed mice.
Conclusions
Our results indicate that hypothermia leads to platelet activation in the spleen via the upregulation of vWF, and this activation causes hypercoagulability after rewarming.
Keywords: hypothermia, microthrombosis, platelet activation, spleen, von Willebrand factor
Essentials.
It remains unclear whether hypothermia enhances or inhibits thrombosis.
We investigated platelet and hemostasis changes in a mouse hypothermia model.
Hypothermia resulted in the activation of platelets in the spleen.
A hypercoagulable state occurred when the body was rewarmed.
1. INTRODUCTION
Platelet activation influences hemostasis and prevents severe bleeding. However, overactivation of platelets often causes thrombotic disease. Hence, platelet activation is tightly controlled under physiological conditions. Platelet homeostasis is sensitive to low temperatures,1, 2 and the rate of platelet activation increases when platelet‐rich plasma is stored at 4°C.3, 4 Furthermore, transfusion of platelet‐rich plasma stored at 4°C enhances beneficial clotting activity.5, 6, 7 Various mechanisms have been proposed to explain this cold‐induced platelet activation.8, 9, 10, 11, 12, 13
Both in vitro and in vivo exposure to low temperatures alter platelet activity and hemostasis; this hypothermia can be therapeutically induced or accidental. Although mild therapeutic hypothermia is a standard treatment component for patients resuscitated from cardiac arrest,14, 15 it also causes coagulation disorders.16, 17 For example, platelet degranulation and dysfunction are triggered by intraoperative hypothermic circulatory arrest.18, 19 In contrast to therapeutic hypothermia, accidental hypothermia is an involuntary decrease in core body temperature to <35°C due to exposure to snow, wind, water, or high altitude. Accidental hypothermia is classified as mild (core temperature 32°C‐35°C), moderate (28°C‐32°C), or severe (<28°C).20 In therapeutic or accidental hypothermia, although platelets are not exposed to a temperature as low as 4°C, coagulation disorders are induced by body temperatures of 28°C‐35°C.14, 15, 16, 17, 18, 19, 20
The mechanism of hypothermia‐induced platelet activation and hemostasis is controversial, with two conflicting mechanisms proposed. The first suggests that hypothermia promotes platelet margination via an increasing hematocrit, which changes platelet shape, reduces blood flow, and increases the expression of adhesion molecules, resulting in thrombosis.21 The second suggests that hypothermia inhibits the enzymatic activities involved in the coagulation cascade.22 Indeed, case studies show that hypothermia induces both a hypercoagulable state and a hemorrhagic tendency.23, 24 Thus, it remains unclear whether hypothermia enhances or inhibits coagulation.
During therapeutic hypothermia, the body temperature reduction is gradual and is closely monitored and controlled25, 26, 27; thus, the alteration of platelet function is normally reversible after the hypothermic state is reversed.4, 28, 29 Conversely, in accidental severe hypothermia, the decrease in body temperature may be rapid and more severe, and the progression and reversibility of hypothermia vary depending on the situation, making the pathophysiological details more difficult to understand. To investigate platelet bioactivity and hemostasis during deep hypothermia, we considered animal models to be more suitable than clinical observations. The characterization of molecular dynamics in thrombosis and hemostasis during hypothermia will contribute to the development of treatment protocols for accidental hypothermia. In the present study, we investigated the platelet and clotting activities in deep hypothermia in a mouse model.
2. MATERIALS AND METHODS
2.1. Animal models
The experimental procedures were approved by the animal experiments committee of Asahikawa Medical University, and all other methods were performed in accordance with the relevant guidelines and regulations. We divided 8‐week‐old male C57Bl/6 mice into six groups (n = 10 in each group; Figure 1): normal control mice (C‐mice), mice euthanized immediately after hypothermia treatment (H‐mice), splenectomized mice that did not undergo hypothermia treatment (SNC‐mice), splenectomized mice that underwent hypothermia treatment (SNH‐mice), mice that underwent hypothermia treatment and rewarming (HR‐mice), and splenectomized mice that underwent hypothermia treatment and rewarming (SNHR‐mice). Splenectomized mice were kept under normal conditions for 4 weeks to recover from surgery before undergoing further treatments. For the hypothermia treatment, the mice were kept at −20°C under general anesthesia, with the rectal temperature monitored using a digital thermometer. Preliminary experiments revealed that cardiac arrest was induced at rectal temperatures of 11°C‐13°C. Therefore, when the rectal temperature decreased to 15°C, whole blood and tissue samples were immediately collected from the H‐mice, which were then euthanized. The other hypothermic group (HR‐mice and SNHR‐mice) were rewarmed by an electric heating pad and then kept in normal conditions for 24 h before sample collection and euthanasia. The whole blood was mixed with 3.2% sodium citrate solution in a 9:1 ratio.
Figure 1.
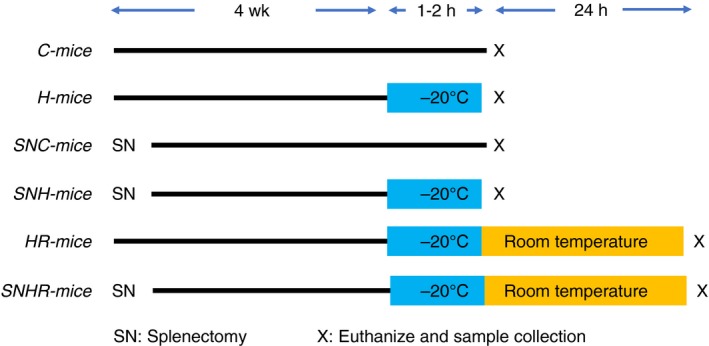
The schema of the experimental design. C, control; H, hypothermic; R, rewarmed. SN, splenectomized
2.2. Histopathological analysis
Formalin‐fixed, paraffin‐embedded tissue samples were prepared in accordance with a standard protocol. We performed immunohistochemical staining for CD62P as a marker of activated platelets, and for CD61 as a marker of total platelets. For the immunohistochemical analysis, 3‐μm‐thick paraffin sections were sequentially treated before application with the primary antibody in the following way: deparaffinization, rehydration, quenching of endogenous peroxidase, and antigen retrieval. Sections were then incubated with primary antibody for CD62P (Novus Biologicals, Littleton, CO) or anti‐CD61 (Cell Signaling Technology, Danvers, MA), followed by detection with horseradish peroxidase‐conjugated secondary antibody for mice or rabbits (Vector Labs, Burlingame, CA). The CD62P‐positive area in splenic red pulp was quantified. Representative pictures of the splenic red pulp from each mouse were taken under ×400 magnification. After obtaining each CD62P‐positive area (square micrometers) using ImageJ software, the ratio of each area to the average area in C‐mice was calculated. Martius Scarlet Blue staining was performed to detect microthrombi.30 The percentage of thrombus‐positive renal glomeruli with respect to the total number of renal glomeruli (one kidney section contains approximately 150 glomeruli) was counted in each kidney under ×400 magnification.
2.3. Complete blood cell count and flow cytometry
Platelet count (PLT) was performed using an automated blood counter (XN‐3100; Sysmex, Kobe, Japan). Whole blood was incubated with fluorescein isothiocyanate‐conjugated anti‐CD61 antibody and phycoerythrin‐conjugated anti‐CD62P antibody (Thermo Fisher Scientific, Waltham, MA). Each fluorescence‐positive platelet was enumerated using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ). The percentage of CD62P/CD61 double‐positive platelets with respect to the number of CD61‐positive platelets was calculated as an indicator of the number of activated platelets in the peripheral blood.
2.4. Scanning electron microscopy
Splenic tissue samples were fixed in 2.5% glutaraldehyde, cut into small blocks, and fixed in 1% osmium for 1 h. The samples were then processed via sequential alcohol dehydration and infiltrated with t‐butyl alcohol. After freezing, the tissues were vacuum‐dried and coated with ion sputter (Hitachi E‐1030; Hitachi, Tokyo, Japan) for analysis with a scanning electron microscope (SEM S‐4100; Hitachi).
2.5. Coagulation assay
Prothrombin time (PT) and activated partial thromboplastin time (APTT) were analyzed using an EnSpire Multimode Plate Reader (PerkinElmer, Waltham, MA).31
2.6. Enzyme‐linked immunosorbent assay
Pro‐platelet basic protein (PPBP), platelet factor 4 (PF4), von Willebrand Factor (vWF), and D‐dimer levels were analyzed using enzyme‐linked immunosorbent assay (ELISA) kits from Abcam (Cambridge, UK) for PPBP, R&D Systems (Minneapolis, MN) for PF4, and LifeSpan BioSciences (Seattle, WA) for vWF and D‐dimer.
2.7. Real‐time polymerase chain reaction
Total RNA was isolated from the spleen and reverse transcribed. Real‐time polymerase chain reaction (RT‐PCR) was performed using the ABI 7300 system (Thermo Fisher Scientific) with TaqMan probes for mouse Vwf messenger RNA (mRNA), and for 18S ribosomal RNA as an internal control. Each threshold cycle was obtained, and the double‐delta threshold cycle method was used to calculate the expression values.
2.8. Western blot analysis
The tissue samples underwent lysis in SDS‐buffer and separation in polyacrylamide‐sodium dodecyl sulfate (SDS) gel and were electrotransferred to nitrocellulose membranes. The membranes were then probed with rabbit anti‐vWF (Abcam) or mouse anti‐Actin antibody (BD Bioscience). The membranes were incubated with the respective horseradish peroxidase‐conjugated antirabbit or antimouse immunoglobulin G secondary antibodies (R&D Systems). Antibody binding was visualized using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). The quantification was performed by densitometric measurement using ImageJ software.
2.9. Statistical analysis
The differences in the experimental values were analyzed via the Student paired t test or one‐way analysis of variance followed by the Tukey‐Kramer method.
3. RESULTS
3.1. Platelet status in the spleen
There were only two or three CD62P‐positive platelet adhesions per blood vessel in the liver, kidneys, heart, lungs, and skin of C‐mice and H‐mice (Figure 2A). The splenic red pulp of H‐mice had a 7‐fold increase in the area of CD62P‐positive platelet adhesions compared with C‐mice, but no significant difference in the amount of CD61‐positive platelets (Figure 2B). H‐mice spleens contained platelets with extended pseudopods (Figure 2C). The plasma levels of PPBP and PF4 (biomarkers of platelet degranulation) were significantly higher in H‐mice compared with C‐mice (Figure 2D).
Figure 2.
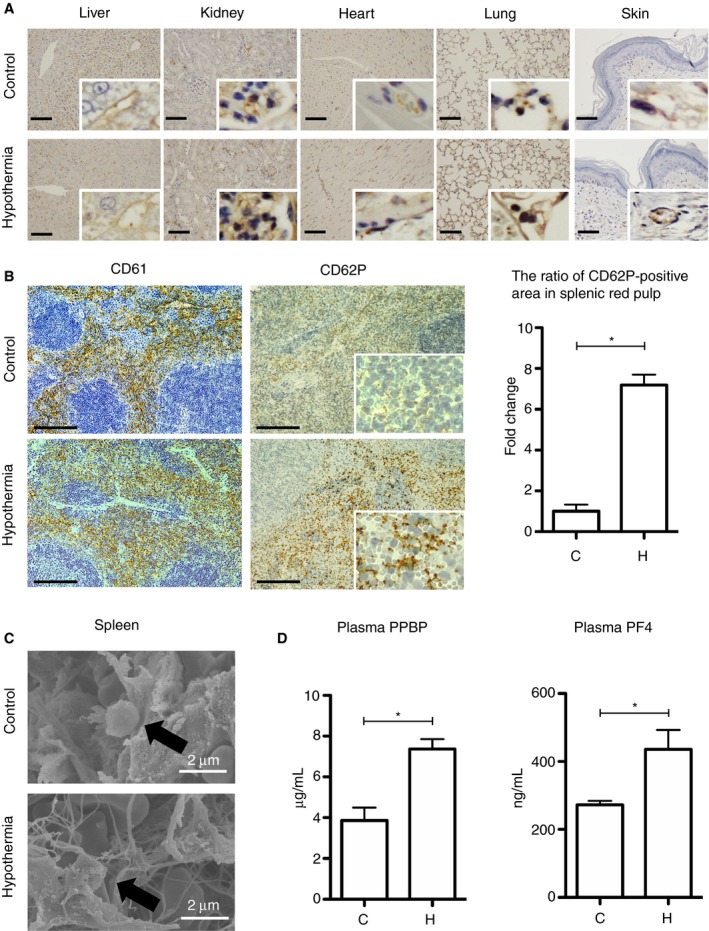
Platelet status in the tissues of mice exposed to hypothermia. A, Immunohistochemical staining for CD62P in the liver, kidneys, heart, lungs, and skin. The scale bars indicate 50 μm. B, Left: Immunohistochemical staining for CD61 and CD62P in the spleen. The scale bars indicate 100 μm. Right: The ratio of the CD62P‐positive area in splenic red pulp (n = 10 per experimental group, *P < .05). C, Scanning electron microscopy in the spleen. D, The levels of PPBP and PF4 in mice plasma (n = 10 per experimental group, *P < .05). The error bar indicates the SD. C, control; H, hypothermic; NS, no significance; PF4, platelet factor 4; PPBP, pro‐platelet basic protein
3.2. Platelet status in the peripheral blood
The trapping of aberrant or aged platelets flowing in the peripheral blood is an important role of splenic red pulp. We, therefore, tried to ascertain whether the appearance of activated platelets in the spleen was due to aberrant activation of platelets in the peripheral blood. Complete blood count revealed no significant differences between C‐mice and H‐mice in the PLT, platelet distribution width, mean platelet volume, platelet large cell ratio, or plateletcrit (Figure 3A). Furthermore, flow cytometry for the detection of CD62P/CD61 double‐positive platelets in the peripheral blood did not reveal any significant differences between C‐mice and H‐mice (Figure 3B).
Figure 3.
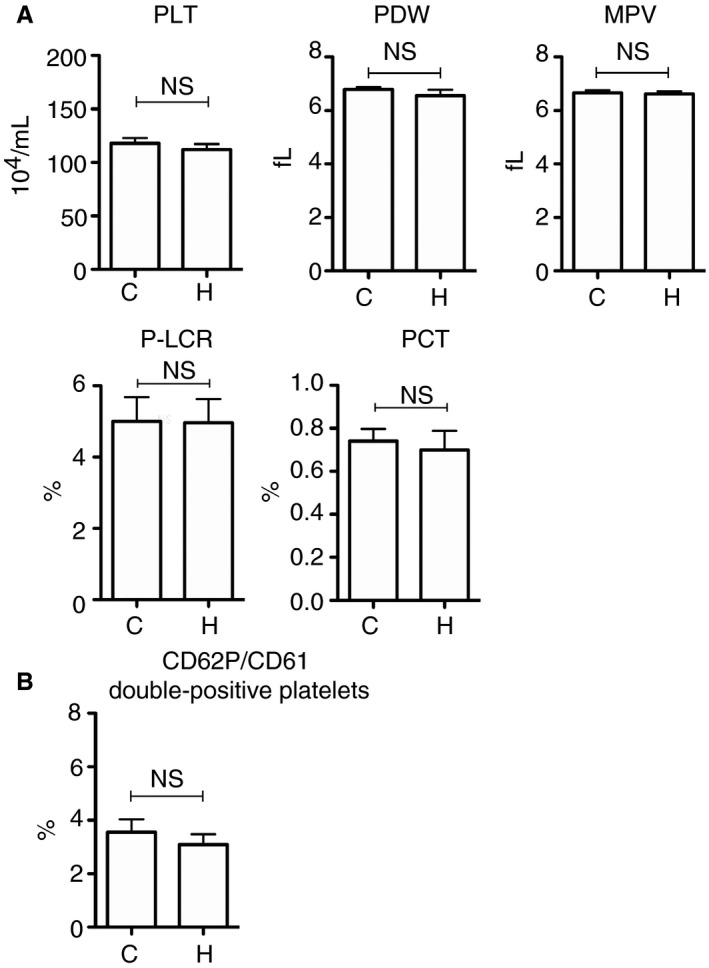
Platelet status in the peripheral blood of hypothermic mice. A, Platelet parameters were determined via complete blood count (n = 10 per experimental group). B, The percentage of CD62P/CD61 double‐positive platelet in the peripheral blood (n = 10 per experimental group). The error bar indicates the SD. C, control; H, hypothermic; MPV, mean platelet volume; NS, no significance; PCT, plateletcrit; PDW, platelet distribution width; P‐LCR, platelet large cell ratio; PLT, platelet count
3.3. The importance of the splenic tissue environment in hypothermia‐induced platelet activation
The results discussed suggested that the platelets that were physiologically pooled in the splenic red pulp might be activated in hypothermic mice. To clarify whether platelet activation was confined to the spleen in hypothermic mice, we performed the hypothermic experiments using splenectomized mice (SNC‐mice and SNH‐mice). Complete blood count revealed no significant differences between SNC‐mice and SNH‐mice in the number or morphology of peripheral platelets (Figure 4A). Flow cytometry for CD62P/CD61 double‐positive platelets in the peripheral blood revealed no significant difference between SNC‐mice and SNH‐mice (Figure 4B). Immunohistochemical staining for CD62P revealed only a few platelet adhesions in the liver, kidneys, heart, lungs, and skin of splenectomized mice (Figure 4C). Additionally, there were no significant differences in the plasma PPBP and PF4 levels of SNC‐mice versus SNH‐mice (Figure 4D).
Figure 4.
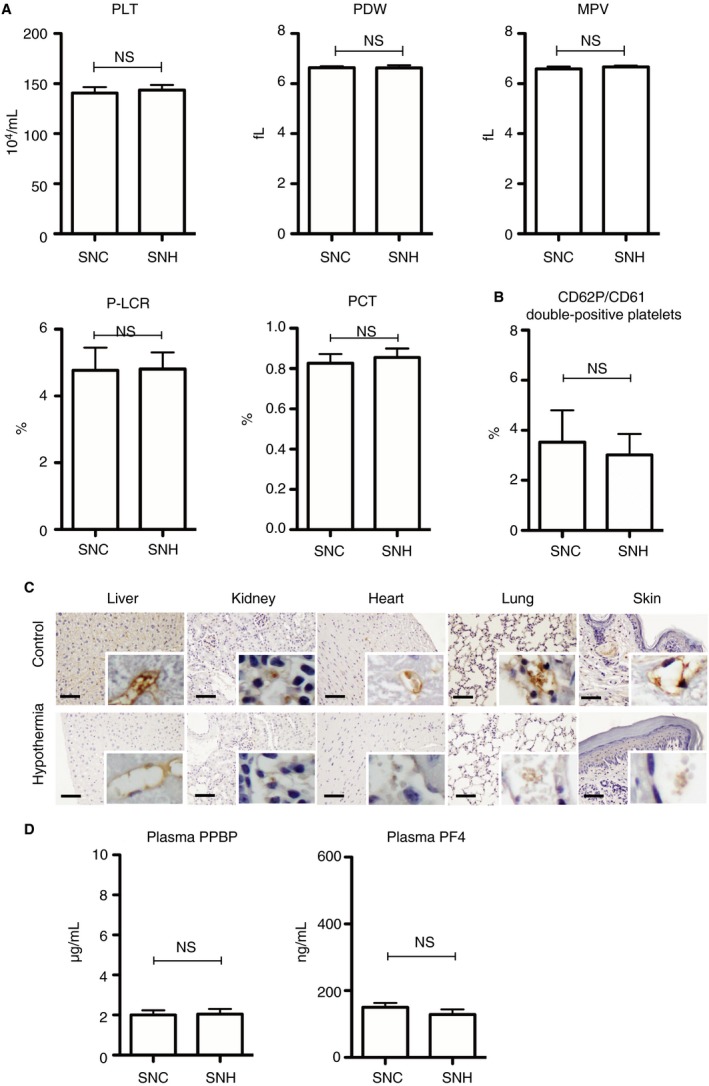
Platelet status in the splenectomized hypothermic mice. A, Platelet parameters were determined via complete blood count (n = 10 per experimental group). B, CD62P/CD61 double‐positive platelets in the peripheral blood (n = 10 per experimental group). C, Immunohistochemical staining for CD62P in the liver, kidneys, heart, lungs, and skin. The scale bars indicate 50 μm. D, PPBP, and PF4 levels in plasma (n = 10 per experimental group). The error bar indicates the SD. C, control; H, hypothermic; MPV, mean platelet volume; NS, no significance; PCT, plateletcrit; PDW, platelet distribution width; PF4, platelet factor 4; P‐LCR, platelet large cell ratio; PLT, platelet count; PPBP, pro‐platelet basic protein; SN, splenectomized
3.4. von Willebrand factor expression in the spleen and plasma under hypothermic conditions
von Willebrand factor plays an important role in platelet activation and adhesion to vascular endothelial cells. Thus, we evaluated the expression levels of mouse Vwf mRNA and the amount of vWF protein. Real‐time polymerase chain reaction demonstrated upregulation of Vwf mRNA expression in the spleens of H‐mice compared with C‐mice (Figure 5A). Similarly, Western blot analysis revealed that the vWF protein expression was elevated in the spleens of H‐mice compared with C‐mice (Figure 5B). There was significant elevation of vWF protein expression in the spleens of H‐mice versus C‐mice, while similar elevations were not observed in the lungs, liver, and kidneys (Figure 5C). In addition, ELISA demonstrated that H‐mice had significantly higher plasma vWF levels than C‐mice (Figure 5C). However, the plasma vWF levels of SNH‐mice did not significantly differ from those of SNC‐mice (Figure 5C).
Figure 5.
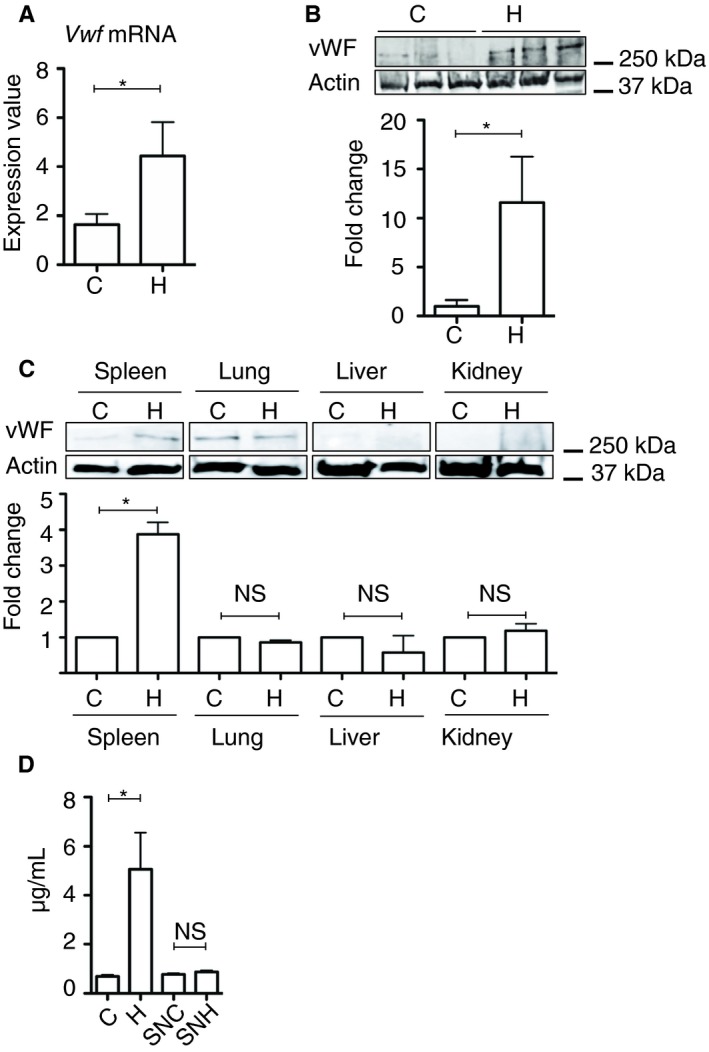
von Willebrand factor gene expression analysis in the spleen and the plasma. A, Real‐time polymerase chain reaction analysis for Vwf mRNA in the spleen (n = 10 per experimental group). B, Western blot analysis for vWF protein in the spleen (n = 6 per experimental group, *P < .05). C, Western blot analysis for vWF protein in the spleen, lung, liver, and kidney (n = 5 per experimental group, *P < .05). D, Plasma levels of vWF protein in each experimental mouse analyzed by ELISA (n = 10 per experimental group, *P < .05). The error bar indicates the SD. C, control; H, hypothermic; NS, no significance; SN, splenectomized; vWF, von Willebrand factor
3.5. Coagulation and fibrinolysis in hypothermic mice
To assess whether the platelet degranulation in the spleen affected the coagulation and fibrinolysis, we evaluated the D‐dimer level (fibrin degradation products), PT, and APTT. There was no significant difference in the plasma D‐dimer level of H‐mice versus C‐mice (Figure 6A). Although the PT did not significantly differ between C‐mice and H‐mice, the APTT was significantly prolonged in H‐mice and SNH‐mice compared with C‐mice and SNC‐mice, respectively (Figure 6B).
Figure 6.
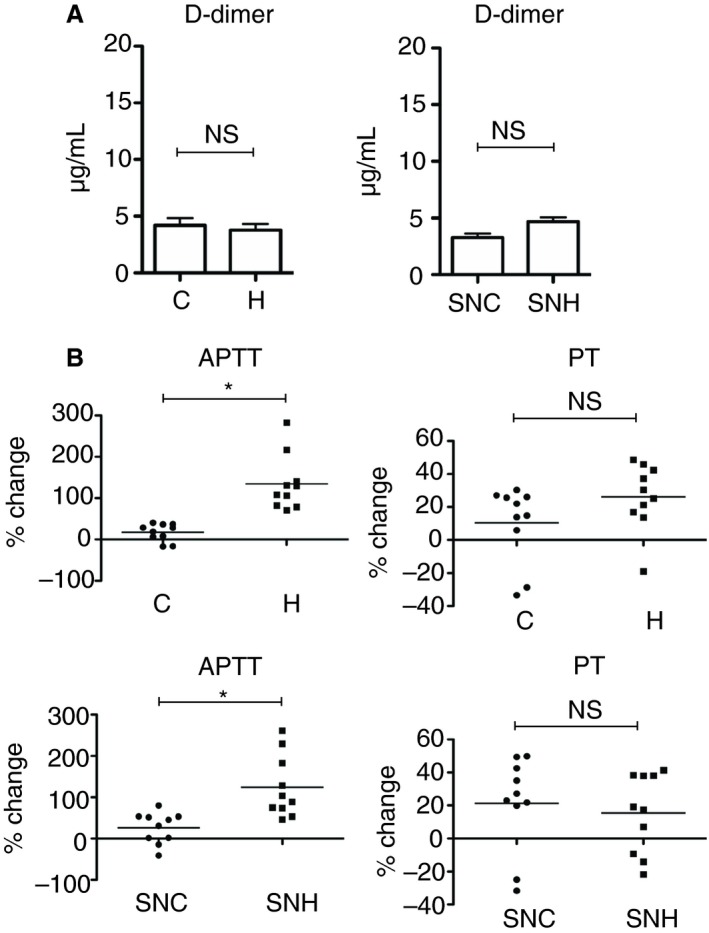
Coagulation activity in hypothermic mice. A, Plasma D‐dimer levels analyzed by ELISA (n = 10 per experimental group, *P < .05). B, APTT and PT in plasma (n = 10 per experimental group, *P < .05). The error bar indicates the SD. APTT, activated partial thromboplastin time; C, control; ELISA, enzyme‐linked immunosorbent assay; H, hypothermic; NS, no significance; PT, prothrombin time; SN, splenectomized
3.6. Hypercoagulation status after rewarming
Twenty‐four hours after rewarming, plasma D‐dimer concentrations were increased in HR‐mice, but not in SNHR‐mice (Figure 7A). Martius Scarlet Blue staining revealed microthrombi in the glomeruli of HR‐mice (an average of 2.3% of glomeruli contained thrombi in five mice) but not in C‐mice, SNC‐mice, and SNHR‐mice (Figure 7B). The APTT and PT were prolonged in HR‐mice, but not in SNHR‐mice (Figure 7C).
Figure 7.
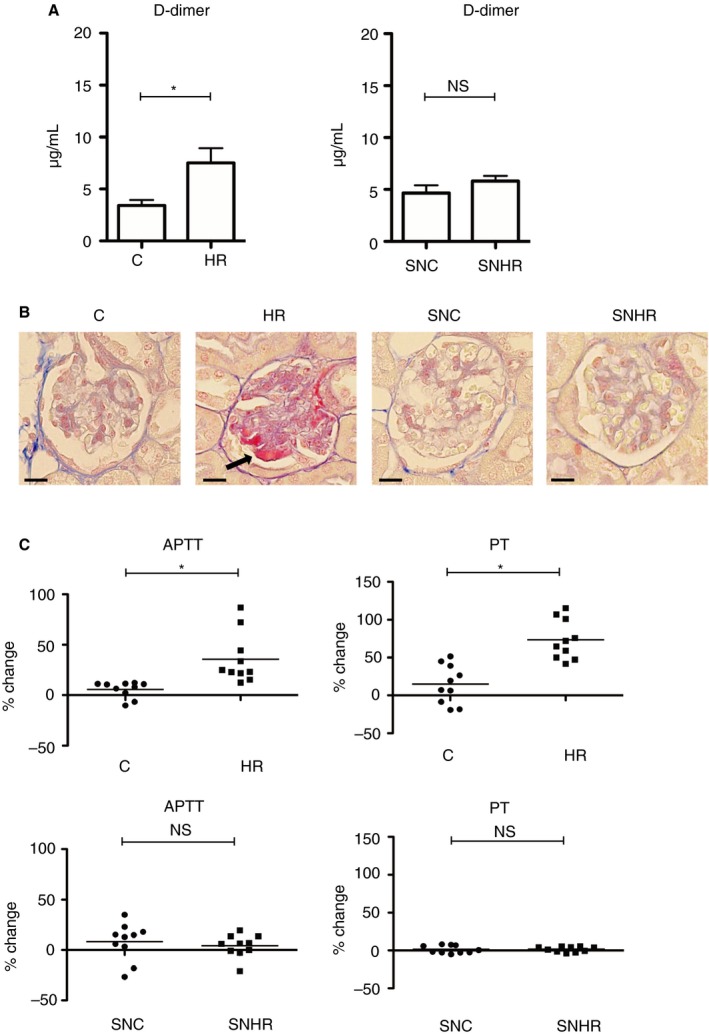
Effect of rewarming on the coagulation state in hypothermic mice. A, D‐dimer levels analyzed by ELISA (n = 10 per experimental group, *P < .05). B, Representative microthrombosis in renal glomeruli stained with Martius Scarlet Blue. In HR‐mice, glomerular thrombi are stained red. The scale bars indicate 20 μm. C, APTT and PT of mice plasma (n = 10 per experimental group, *P < .05). The error bar indicates the SD. APTT, activated partial thromboplastin time; C, control; H, hypothermic; NS, no significance; PT, prothrombin time; R, rewarmed; SN, splenectomized
4. DISCUSSION
Previous studies report platelet sequestration in the spleen and liver along with peripheral platelet reduction in hypothermic animals.32, 33 However, the biological significance of these phenomena and the function of platelets in this specific context remain unclear. Furthermore, these previous studies involved relatively mild hypothermic conditions.32, 33 In the present study, we exposed the mice to severe hypothermia and discovered new information about platelet activity in hypothermia. The mice exposed to deep hypothermia had an abundance of CD62P‐positive activated platelets in the splenic red pulp, while the amount of CD61‐positive platelets was unchanged. Moreover, the plasma PPBP and PF4 levels increased after exposure to low temperatures, indicating platelet degranulation; however, the peripheral platelet parameters (PLT, mean platelet volume, platelet distribution width, and number of CD62P/CD61 double‐positive platelets) did not significantly differ from thyose of control mice. Furthermore, the plasma PPBP and PF4 levels did not increase in splenectomized mice after exposure to low temperatures, indicating that the splenic tissue environment was necessary for hypothermia‐induced platelet activation. One‐third of the platelets are stored in the spleen under physiological conditions.34 Thus, our findings suggest that severe hypothermia did not affect peripheral platelets but caused activation and the degranulation of the platelets physiologically pooled in the splenic red pulp.
Although several other studies showed a reduction in the peripheral platelets after hypothermia,32, 33 we did not observe this phenomenon. This discrepancy may be due to the difference between studies in the severity of hypothermia. In our severe hypothermia model, the body core temperature was markedly decreased, while the other studies used only a mild reduction in body temperature. We kept the mice at −20°C under general anesthesia to mimic severe accidental hypothermia in humans. It took approximately 1 h for the rectal temperature of the mice to decrease to 15°C. We then collected the peripheral blood samples immediately after the rectal temperatures decreased to 15°C, as it was difficult to keep the mice alive past this timepoint. We hypothesize that this short period of severe hypothermia was not long enough to cause splenic platelet sequestration, but that the activation of the platelet pool in the spleen occurred prior to the sequestration of peripheral platelets.
Many reports have described the interactions between vWF and platelet receptors, glycoprotein Ib‐IX‐V, and αIIbβ3 integrin‐promoted primary platelet adhesion and aggregation.35, 36, 37, 38 In our splenic tissue analysis, hypothermia enhanced vWF expression in both mRNA and protein. However, there was no significant hypothermia‐induced vWF expression in the other organs we tested. Furthermore, we observed upregulation of plasma vWF in hypothermic mice, but not in asplenic hypothermic mice, indicating that hypothermia‐induced platelet activation is caused by overproduction of vWF in the spleen under hypothermic conditions. These novel findings provide important information about hypothermia‐induced platelet abnormalities in the spleen. However, the mechanisms involved in the regulation of vWF expression under hypothermic conditions require further investigation.
Upon activation, platelets release various substances from their intracellular granules that contribute to coagulation.39, 40, 41, 42, 43, 44 For example, plasma PPBP and PF4 reportedly activate coagulation.45, 46, 47, 48 Although we suspect that these substances were released into the plasma of hypothermic mice, we did not detect significant thrombosis or plasma D‐dimer elevation in mice euthanized immediately after the hypothermia treatment. This suggests that platelet activation in the hypothermic spleens did not cause thrombosis when the body temperature was low, even when active platelet‐derived substances were released into the plasma.
Some clinical observations have demonstrated that low temperatures cause prolonged PT and APTT.49, 50 We also observed prolonged APTT in the plasma collected from mice immediately after the hypothermia treatment, regardless of the presence or absence of splenic tissue where the platelets were being activated, indicating that the hypothermia itself inhibited the intrinsic pathway of the coagulation cascade. Some in vitro studies have shown that cold storage of plasma results in the reduction of coagulation factors.51, 52, 53 However, the impact of body temperature reduction on each coagulation factor needs further investigation.
The rewarmed non‐splenectomized mice had an increased plasma D‐dimer concentration and microthrombosis in renal glomeruli, indicating a hypercoagulable state; these changes were not observed in the rewarmed asplenic mice. The rewarmed non‐splenectomized mice also had prolonged PT and APTT, while the rewarmed asplenic mice did not. As the prolongation of PT and APTT in the rewarmed non‐splenectomized mice was accompanied by D‐dimer elevation and microthrombosis, this suggests that the coagulation factors had been consumed. Furthermore, these observations indicate that the coagulation substances released by platelets during hypothermia in the spleen upregulated the coagulation cascade and resulted in microthrombosis when the body was rewarmed. Presumably, the activity of coagulants released during platelet degranulation was inhibited when the body temperature was low but was reactivated by rewarming.
In conclusion, the main findings of the present study were that severe hypothermia increased vWF production in the spleen, which induced platelet activation and degranulation. This phenomenon resulted in microthrombosis when the body was rewarmed. Our results provide novel information, indicating that the activation of platelets in the splenic pool is the key biological event in the hypothermia‐induced hypercoagulable state. Irrespective of whether hypothermia is therapeutic or accidental, rewarming is necessary for recovery. Therefore, it is important to keep in mind that platelet activation in the splenic pool leads to microthrombosis upon rewarming.
CONFLICT OF INTEREST
The authors have no conflicts of interest regarding the contents of this article.
AUTHOR CONTRIBUTIONS
K. H. and H. T. designed and performed all experimental procedures and wrote the manuscript. K. H., S. I., K. Ok., and M. A. performed data analysis. H. T. and K. Og. supervised and reviewed this manuscript. H. S. and K. S. contributed to the acquisition of funding.
Horioka K, Tanaka H, Isozaki S, et al. Hypothermia‐induced activation of the splenic platelet pool as a risk factor for thrombotic disease in a mouse model. J Thromb Haemost. 2019;17:1762–1771. 10.1111/jth.14555
Manuscript handled by: Andreas Greinacher
Final decision: Andreas Greinacher, 19 June 2019
REFERENCES
- 1. Wang X, Fan Y, Shi R, Li J, Zhao S. Quality assessment of platelets stored in a modified platelet additive solution with trehalose at low temperature (10 degrees C) and in vivo effects on rabbit model of thrombocytopenia. Platelets. 2015;26:72–9. [DOI] [PubMed] [Google Scholar]
- 2. Winokur R, Hartwig JH. Mechanism of shape change in chilled human platelets. Blood. 1995;85:1796–804. [PubMed] [Google Scholar]
- 3. Baimukanova G, Miyazawa B, Potter DR, Gibb SL, Keating S, Danesh A, et al. The effects of 22°C and 4°C storage of platelets on vascular endothelial integrity and function. Transfusion. 2016;56:S52–64. [DOI] [PubMed] [Google Scholar]
- 4. Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Bergmeier W, Mayadas TN, et al. The clearance mechanism of chilled blood platelets. Cell. 2003;112:87–97. [DOI] [PubMed] [Google Scholar]
- 5. Kaufman RM. Uncommon cold: could 4°C storage improve platelet function? Transfusion. 2005;45:1407–12. [DOI] [PubMed] [Google Scholar]
- 6. Nair PM, Pandya SG, Dallo SF, Reddoch KM, Montgomery RK, Pidcoke HF, et al. Platelets stored at 4 degrees C contribute to superior clot properties compared to current standard‐of‐care through fibrin‐crosslinking. Br J Haematol. 2017;178:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wandall HH, Hoffmeister KM, Sorensen AL, Rumjantseva V, Clausen H, Hartwig JH, et al. Galactosylation does not prevent the rapid clearance of long‐term, 4 degrees C‐stored platelets. Blood. 2008;111:3249–56. [DOI] [PubMed] [Google Scholar]
- 8. Berzuini A, Spreafico M, Prati D. One size doesn't fit all: should we reconsider the introduction of cold‐stored platelets in blood bank inventories? F1000Res. 2017;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nair PM, Pidcoke HF, Cap AP, Ramasubramanian AK. Effect of cold storage on shear‐induced platelet aggregation and clot strength. J Trauma Acute Care Surg. 2014;77:S88–93. [DOI] [PubMed] [Google Scholar]
- 10. Gousset K, Tsvetkova NM, Crowe JH, Tablin F. Important role of raft aggregation in the signaling events of cold‐induced platelet activation. Biochim Biophys Acta. 2004;1660:7–15. [DOI] [PubMed] [Google Scholar]
- 11. Marini I, Aurich K, Jouni R, Nowak‐Harnau S, Hartwich O, Greinacher A, et al. Cold storage of platelets in additive solution: the impact of residual plasma in apheresis platelet concentrates. Haematologica. 2019;104:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montgomery RK, Reddoch KM, Evani SJ, Cap AP, Ramasubramanian AK. Enhanced shear‐induced platelet aggregation due to low‐temperature storage. Transfusion. 2013;53:1520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reddoch KM, Pidcoke HF, Montgomery RK, Fedyk CG, Aden JK, Ramasubramanian AK, et al. Hemostatic function of apheresis platelets stored at 4 degrees C and 22 degrees C. Shock. 2014;41(Suppl 1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kowalik R, Szczerba E, Koltowski L, Grabowski M, Chojnacka K, Golecki W, et al. Cardiac arrest survivors treated with or without mild therapeutic hypothermia: performance status and quality of life assessment. Scand J Trauma Resusc Emerg Med. 2014;22:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Q, Miao P, Modi HR, Garikapati S, Koehler RC, Thakor NV. Therapeutic hypothermia promotes cerebral blood flow recovery and brain homeostasis after resuscitation from cardiac arrest in a rat model. J Cereb Blood Flow Metab. 2018;271678X18773702 10.1177/0271678X18773702 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gando S, Otomo Y. Local hemostasis, immunothrombosis, and systemic disseminated intravascular coagulation in trauma and traumatic shock. Crit Care. 2015;19:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–69. [DOI] [PubMed] [Google Scholar]
- 18. Watts DD, Trask A, Soeken K, Perdue P, Dols S, Kaufmann C. Hypothermic coagulopathy in trauma: effect of varying levels of hypothermia on enzyme speed, platelet function, and fibrinolytic activity. J Trauma. 1998;44:846–54. [DOI] [PubMed] [Google Scholar]
- 19. Valeri CR, MacGregor H, Cassidy G, Tinney R, Pompei F. Effects of temperature on bleeding time and clotting time in normal male and female volunteers. Crit Care Med. 1995;23:698–704. [DOI] [PubMed] [Google Scholar]
- 20. Petrone P, Asensio JA, Marini CP. Management of accidental hypothermia and cold injury. Curr Probl Surg. 2014;51:417–31. [DOI] [PubMed] [Google Scholar]
- 21. Van Poucke S, Stevens K, Marcus AE, Lance M. Hypothermia: effects on platelet function and hemostasis. Thromb J. 2014;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reed RL 2nd, Bracey AW Jr, Hudson JD, Miller TA, Fischer RP. Hypothermia and blood coagulation: dissociation between enzyme activity and clotting factor levels. Circ Shock. 1990;32:141–52. [PubMed] [Google Scholar]
- 23. Ruzicka J, Stengl M, Bolek L, Benes J, Matejovic M, Krouzecky A. Hypothermic anticoagulation: testing individual responses to graded severe hypothermia with thromboelastography. Blood Coagul Fibrinolysis. 2012;23:285–9. [DOI] [PubMed] [Google Scholar]
- 24. Debaty G, Moustapha I, Bouzat P, Maignan M, Blancher M, Rallo A, et al. Outcome after severe accidental hypothermia in the French Alps: a 10‐year review. Resuscitation. 2015;93:118–23. [DOI] [PubMed] [Google Scholar]
- 25. Alshimemeri A. Therapeutic hypothermia after cardiac arrest. Ann Card Anaesth. 2014;17:285–91. [DOI] [PubMed] [Google Scholar]
- 26. Cortez E, Panchal AR, Davis J, Zeeb P, Keseg DP. Clinical outcomes in cardiac arrest patients following prehospital treatment with therapeutic hypothermia. Prehosp Disaster Med. 2015;30:452–6. [DOI] [PubMed] [Google Scholar]
- 27. Lin JJ, Lin CY, Hsia SH, Wang HS, Chiang MC, Lin KL. 72‐Hour therapeutic hypothermia improves neurological outcomes in paediatric asphyxial out‐of‐hospital cardiac arrest – an exploratory investigation. Resuscitation. 2018;133:180–6. [DOI] [PubMed] [Google Scholar]
- 28. Ao H, Moon JK, Tashiro M, Terasaki H. Delayed platelet dysfunction in prolonged induced canine hypothermia. Resuscitation. 2001;51:83–90. [DOI] [PubMed] [Google Scholar]
- 29. de Vrij EL, Vogelaar PC, Goris M, Houwertjes MC, Herwig A, Dugbartey GJ, et al. Platelet dynamics during natural and pharmacologically induced torpor and forced hypothermia. PLoS ONE. 2014;9:e93218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fisseler‐Eckhoff A, Muller KM. Lendrum (‐MSB) staining for fibrin identification in sealed skin grafts. Pathol Res Pract. 1994;190:444–8. [DOI] [PubMed] [Google Scholar]
- 31. Tshikudi DM, Tripathi MM, Hajjarian Z, Van Cott EM, Nadkarni SK. Optical sensing of anticoagulation status: towards point‐of‐care coagulation testing. PLoS ONE. 2017;12:e0182491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pina‐Cabral JM, Amaral I, Pinto MM, Guerra E, Paz LH. Hepatic and Splenic platelet sequestration during deep hypothermia in the dog. Pathophysiol Haemos Thromb. 1973;2:235–44. [Google Scholar]
- 33. Pina‐Cabral JM, Ribeiro‐da‐Silva A, Almeida‐Dias A. Platelet sequestration during hypothermia in dogs treated with sulphinpyrazone and ticlopidine–reversibility accelerated after intra‐abdominal rewarming. Thromb Haemost. 1985;54:838–41. [PubMed] [Google Scholar]
- 34. Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of “hypersplenic” thrombocytopenia. J Clin Invest. 1966;45:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jansen AJ, Josefsson EC, Rumjantseva V, Liu QP, Falet H, Bergmeier W, et al. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbalpha metalloproteinase‐mediated cleavage in mice. Blood. 2012;119:1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sandgren P, Hansson M, Gulliksson H, Shanwell A. Storage of buffy‐coat‐derived platelets in additive solutions at 4 degrees C and 22 degrees C: flow cytometry analysis of platelet glycoprotein expression. Vox Sang. 2007;93:27–36. [DOI] [PubMed] [Google Scholar]
- 37. van der Wal DE, Du VX, Lo KS, Rasmussen JT, Verhoef S, Akkerman JW. Platelet apoptosis by cold‐induced glycoprotein Ibalpha clustering. J Thromb Haemost. 2010;8:2554–62. [DOI] [PubMed] [Google Scholar]
- 38. Gitz E, Koopman CD, Giannas A, Koekman CA, van den Heuvel DJ, Deckmyn H, et al. Platelet interaction with von Willebrand factor is enhanced by shear‐induced clustering of glycoprotein Ibalpha. Haematologica. 2013;98:1810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Gaetano G. Historical overview of the role of platelets in hemostasis and thrombosis. Haematologica. 2001;86:349–56. [PubMed] [Google Scholar]
- 40. Chen Y, Yuan YF, Li W. Sorting machineries: how platelet dense granules differ from alpha‐granules. Biosci Rep. 2018;38:BSR20180458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharda A, Flaumenhaft R. The life cycle of platelet granules. F1000Res.[qqq] 2018;7:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mumford AD, Frelinger AL 3rd, Gachet C, Gresele P, Noris P, Harrison P, et al. A review of platelet secretion assays for the diagnosis of inherited platelet secretion disorders. Thromb Haemost. 2015;114:14–25. [DOI] [PubMed] [Google Scholar]
- 43. Heijnen H, van der Sluijs P. Platelet secretory behaviour: as diverse as the granules … or not? J Thromb Haemost. 2015;13:2141–51. [DOI] [PubMed] [Google Scholar]
- 44. Rendu F, Brohard‐Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets. 2001;12:261–73. [DOI] [PubMed] [Google Scholar]
- 45. Sagedal S, Sandvik L, Klingenberg O, Sandset PM. beta‐Thromboglobulin may not reflect platelet activation during haemodialysis with the HeprAN membrane. Scand J Clin Lab Invest. 2017;77:679–84. [DOI] [PubMed] [Google Scholar]
- 46. Schoorl M, Schoorl M, Nube MJ, Bartels PC. Platelet depletion, platelet activation and coagulation during treatment with hemodialysis. Scand J Clin Lab Invest. 2011;71:240–7. [DOI] [PubMed] [Google Scholar]
- 47. Mosnier LO. Platelet factor 4 inhibits thrombomodulin‐dependent activation of thrombin‐activatable fibrinolysis inhibitor (TAFI) by thrombin. J Biol Chem. 2011;286:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Egan K, van Geffen JP, Ma H, Kevane B, Lennon A, Allen S, et al. Effect of platelet‐derived beta‐thromboglobulins on coagulation. Thromb Res. 2017;154:7–15. [DOI] [PubMed] [Google Scholar]
- 49. Darlington DN, Kremenevskiy I, Pusateri AE, Scherer MR, Fedyk CG, Kheirabaldi BS, et al. Effects of in vitro hemodilution, hypothermia and rFVIIa addition on coagulation in human blood. Int J Burns Trauma. 2012;2:42–50. [PMC free article] [PubMed] [Google Scholar]
- 50. Martini WZ, Cortez DS, Dubick MA, Park MS, Holcomb JB. Thrombelastography is better than PT, aPTT, and activated clotting time in detecting clinically relevant clotting abnormalities after hypothermia, hemorrhagic shock and resuscitation in pigs. J Trauma. 2008;65:535–43. [DOI] [PubMed] [Google Scholar]
- 51. Kuta P, Hauck‐Dlimi B, Strobel J, Zimmermann R, Eckstein R. Quality of clotting factor activity in fresh frozen plasma at thaw with a microwave system and after storage at 4 degrees C for 48 hours. Clin Lab. 2016;62:987–91. [DOI] [PubMed] [Google Scholar]
- 52. Lokhandwala PM, O'Neal A, Patel EU, Brunker PAR, Gehrie EA, Zheng G, et al. Hemostatic profile and safety of pooled cryoprecipitate up to 120 hours after thawing. Transfusion. 2018;58:1126–31. [DOI] [PubMed] [Google Scholar]
- 53. Kim YA, Lewandrowski KB, Lucien FA, Van Cott EM. The effects of transport temperature and time on routine and specialized coagulation assays. Blood Coagul Fibrinolysis. 2018;29:184–8. [DOI] [PubMed] [Google Scholar]


