Figure 1.
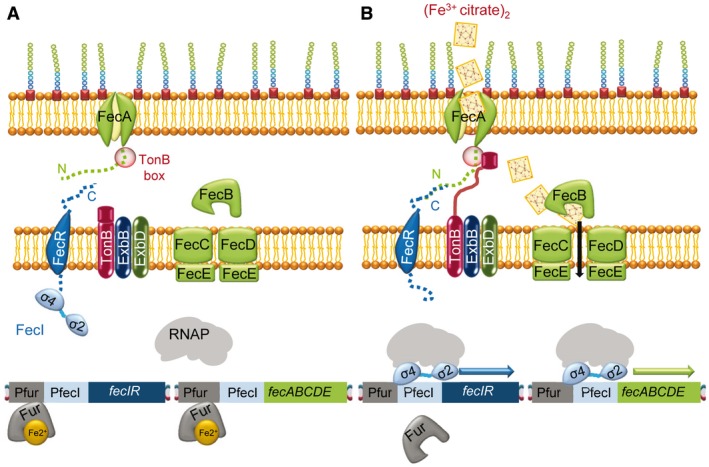
The E. coli CSS Fe3+‐citrate transport is regulated by the FecR/FecI system. A. In the absence of Fe3+‐citrate and in the presence of Fe2+‐Fur, the repressor binds to the region upstream of the operons fecIR and fecABCDE. Moreover, the FecR anti‐sigma factor sequesters the FecI ECF sigma factor by interaction of the N‐terminal region of the anti‐sigma factor and the σ4 domain of the ECF sigma factor, preventing transcription of these two operons. B. Signaling pathway in the presence of Fe3+‐citrate and under low iron availability. The Fur repressor is not bound to Fe2+ and cannot bind DNA. The outer membrane protein receptor FecA suffers conformational changes, interacts with TonB through the TonB box domain, and allows the transport of the substrate to the periplasm, and through FecB and the FecCDE transporter to the cytoplasm. FecA changes also allow interaction between the FecA N‐terminal region and the C‐terminal region of the anti‐sigma factor FecR, releasing the ECF sigma factor FecI. This sigma factor can now up‐regulate the transcription of fecIR and fecABCDE after recruiting the core RNAP.
