Abstract
Objectives:
Between 2003 and 2013, the rate of neonatal abstinence syndrome (NAS)—a postnatal drug withdrawal syndrome—in Tennessee increased approximately 10-fold. NAS surveillance is relatively new, and underestimation associated with surveillance has not been described. We compared data from the Tennessee NAS public health surveillance system (TNSS) with a second source of NAS data, hospital discharge data system (HDDS), and estimated the true number of infants with NAS using capture-recapture methods.
Methods:
We obtained NAS data on cases of NAS among Tennessee infants from TNSS and HDDS from January 1, 2013, through December 31, 2016. We matched cases of NAS identified in TNSS to cases identified in HDDS. We estimated the true number of infants with NAS by using the Lincoln-Peterson estimator capture-recapture methodology.
Results:
During the study period, 4070 infants with NAS were reported to TNSS, and 5321 infants with NAS were identified in HDDS; 2757 were in both data sets. Using capture-recapture methods, the total estimated number of infants with NAS during the study period was 7855 (annual mean = 1972; estimated range = 1531-2427), which was 93% more than in TNSS and 48% more than in HDDS. Drugs used for the medication-assisted treatment of substance use disorder were the most commonly reported substances associated with NAS (n = 2389, 59%).
Conclusions:
TNSS underestimated the total burden of NAS based on the capture-recapture estimate. Case-based public health surveillance is important for monitoring the burden of and risk factors for NAS and helping guide public health interventions.
Keywords: neonatal abstinence syndrome, surveillance system, medication-assisted treatment, MAT, capture-recapture
As the current opioid crisis grows, neonatal abstinence syndrome (NAS), a postnatal drug withdrawal syndrome, is an increasing public health problem in Tennessee and nationally.1 Access to opioids is widespread; for example, in 2016, approximately 108 opioid prescriptions per 100 Tennessee residents were reported.2 Between 2003 and 2013, Tennessee hospital discharges of infants born with NAS increased approximately 10-fold, from 1.6 per 1000 live births in 2003 to 16.6 per 1000 live births in 2013 (Tennessee Department of Health, unpublished hospital discharge data, 2013). In 2013, 427 of 921 (46.4%) NAS cases were associated with medication-assisted treatment (MAT).3
In response to the increasing number of infants with NAS, Tennessee made NAS a reportable condition in 2013.3 Surveillance is important for describing the incidence and trends in NAS and the effect that risk factors such as maternal substance use and treatment have on newborns. Underreporting and uncertainty about the true number of cases is a limitation of public health surveillance systems,4 and few studies have evaluated NAS surveillance. Studies have primarily described NAS cases, social determinants associated with cases, and geographic trends, but they have not explored how completely NAS surveillance is able to ascertain cases.5-8
Infants with NAS have been reported to develop cognitive, language, and motor-function difficulties. A history of NAS is associated with lower test scores compared with children who did not have NAS, and it increases the odds of being diagnosed with a learning disability or having a speech or language impairment.9-11 Long-term adverse effects may differ depending on the substance causing NAS.12-14 Some evidence suggests that infants with MAT-associated NAS do not develop physical, language, or cognitive delays more frequently than unexposed infants,13 and MAT with buprenorphine or methadone is preferred over no treatment because it is associated with improved maternal and neonatal outcomes.15-17 Therefore, tracking MAT-associated NAS is an important element of the public health response to the opioid crisis.
The objectives of this study were to (1) compare data from the Tennessee NAS public health surveillance system (TNSS) with data from the hospital discharge data system (HDDS) to estimate the true number of infants with NAS by using capture-recapture methods and (2) assess the proportion of cases associated with MAT.
Methods
We obtained NAS data from January 1, 2013, through December 31, 2016, for Tennessee infants from TNSS and HDDS. TNSS is a case-based, passive surveillance system operated by the Tennessee Department of Health. Medical providers are required to report to public health authorities any infant showing signs of withdrawal from in utero exposure to drugs.3 TNSS data include date of birth, sex, last 4 digits of the medical record number, names of the reporting hospital and birth hospital, and mother’s county and state of residence.
In 2016, TNSS started collecting data on NAS severity. Severe NAS is defined as requiring pharmacotherapy for withdrawal symptoms; mild NAS is defined as requiring only treatment with environmental modifications. TNSS collects data that support an NAS diagnosis, including a maternal history of using substance(s) known to cause NAS and a maternal or neonatal drug screening test that is positive for a substance known to cause NAS (eg, hair, meconium, or umbilical cord testing positive for an opioid).
Medical providers report the source of maternal exposure for infants with NAS. Nine nonmutually exclusive categories are used: MAT (eg, methadone or buprenorphine), legally prescribed opioid pain reliever (eg, hydrocodone or oxycodone), legally prescribed nonopioid (eg, benzodiazepine), prescription opioid without a prescription (eg, taking someone else’s opioid pain medication), prescription nonopioid without a prescription (eg, taking someone else’s benzodiazepine), a nonprescription substance excluding heroin (eg, cocaine or methamphetamine), heroin, mothers with no known exposures but infants with signs and symptoms consistent with withdrawal, and other.
HDDS is a statewide administrative data source that collects billing data for all-payer inpatient and outpatient hospital discharges. We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 779.5 (drug withdrawal syndrome in a newborn)18 or International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) code P96.1 (neonatal withdrawal symptoms from maternal use of drugs of addiction)19 within 365 days of birth from any of 18 diagnosis code fields to identify NAS cases in HDDS. Variables in HDDS included name, sex, date of birth, county and state of residence, medical record number, hospital, and ICD-9-CM or ICD-10-CM diagnostic codes. Data on infants who received an NAS diagnosis and the maternal medical history are not collected in this database.
We linked cases identified in TNSS to cases identified in HDDS using a 3-step algorithm. We identified exact matches by date of birth, sex, last 4 digits of medical record number, and either birth hospital or transfer hospital (n = 2053). Next, we identified matches using less stringent criteria from the remaining unmatched cases, matching with date of birth, sex, and last 4 digits of medical record number (n = 24) and then those matching by date of birth, sex, and hospital identifier (n = 696). We manually reviewed all matches to eliminate potential duplicates.
We estimated the true number of patients with NAS in Tennessee by using the Lincoln-Peterson estimator capture-recapture method.20 All NAS diagnoses reported in TNSS and HDDS were accepted as accurate for this calculation. We used standard assumptions for capture-recapture estimation: The population was closed, all cases were identified, and each case had an equal chance of occurring in HDDS and a separate but equal chance of occurring in TNSS.20,21
We calculated rates by using 2016 US Census Bureau22 state and county population data and Tennessee Department of Health’s live birth data.23–26 We analyzed geographic distribution in 2016 at the county level. We performed statistical analyses by using SAS version 9.427 and spatial analyses by using ArcMap version 10.3.28 The Centers for Disease Control and Prevention reviewed this activity for human subjects protection and determined it to be nonresearch.
Results
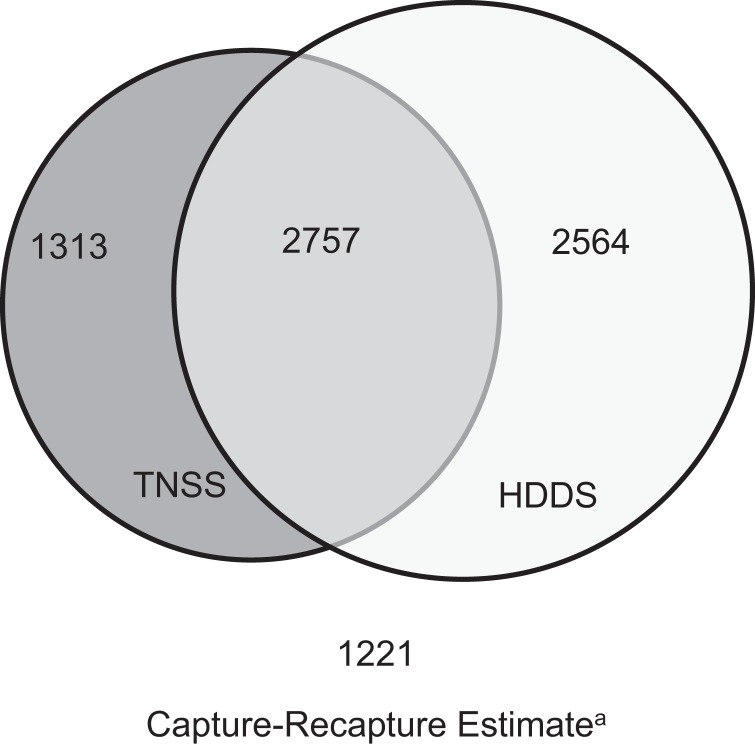
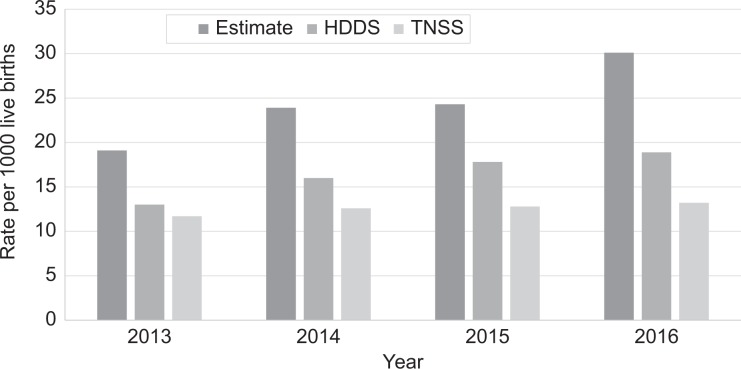
Between 2013 and 2016, a total of 4070 NAS cases were reported to TNSS, and 5321 cases were identified in HDDS, of which 2757 were present in both systems (68% in TNSS, 52% in HDDS) (Figure 1). All cases in TNSS and HDDS had 4 identifying variables available for linking (date of birth, sex, hospital, medical record number). The mean number of cases reported to TNSS annually was 1018 (range = 933-1063), and the mean number of cases in HDDS annually was 1330 (range = 1042-1525); the number of cases in both systems increased from 2013 through 2016. Similarly, rates for TNSS, HDDS, and capture-recapture estimates increased over time (Figure 2). The estimated true number of infants with NAS for the study period, using capture-recapture methods, was 7855 (95% confidence interval [CI], 7739-7971; 93% more than in TNSS and 48% more than in HDDS). The mean annual estimated number of infants with NAS was 1971 (range = 1531-2427).
Figure 1.
Neonatal abstinence syndrome (NAS) cases identified in the Tennessee NAS public health surveillance system (TNSS), hospital discharge database system (HDDS), cases in both data sets, and the capture-recapture estimate, Tennessee, 2013-2016. The capture-recapture estimate was calculated using the Lincoln-Peterson estimator capture-recapture method.20
Figure 2.
Rates per 1000 live births of infants with neonatal abstinence syndrome (NAS) from the Tennessee NAS public health surveillance system (TNSS), hospital discharge database system (HDDS), and capture-recapture estimates, Tennessee, 2013-2016. The capture-recapture estimate was calculated using the Lincoln-Peterson estimator capture-recapture method.20
Significantly more infants with NAS in both databases were male (TNSS: 2204/4070 [54%]; HDDS: 2875/5321 [54%]; P < .001). Infants born with NAS disproportionately resided in northeastern Tennessee, whereas the counties with the lowest rates were in western Tennessee (eg, TNSS NAS 2016 rates per 1000 live births were 114 in Hancock County, 84 in Carter County, 78 in Campbell County, and 0 in Crockett, Decatur, and Hardeman counties). Six hundred ninety-one of 1063 (65%) cases reported to TNSS in 2016 had severe NAS, and 3756 of 4070 (92%) infants were born to women with a history of using a substance known to cause NAS (Table). Drugs used for MAT were the most commonly identified sources of exposure for infants with NAS (n = 2389, 59%). Other maternal exposures were taking prescription opioids without a prescription (289/1063, 27%) and using nonprescription substances other than heroin (881/4070, 22%). Maternal heroin use was reported for 40 of 1063 (4%) infants with NAS.
Table.
Characteristics of infants with neonatal abstinence syndrome (NAS) reported to the Tennessee NAS public health surveillance system (TNSS), 2013-2016
| Characteristic |
Cumulative
No. (%) (n = 4070) |
|---|---|
| NAS severitya | |
| Severe | 691 (65) |
| Mild | 365 (34) |
| Elements used to support making an NAS diagnosisb | |
| Maternal history of using a substance known to cause NAS | 3756 (92) |
| Maternal screening test for a substance known to cause NAS | 2376 (58) |
| Neonatal screening test for a substance known to cause NAS | 2204 (54) |
| Maternal sources of exposureb | |
| Medication-assisted treatment (eg, methadone or buprenorphine) | 2389 (59) |
| Legally prescribed opioid pain reliever (eg, hydrocodone or oxycodone) | 521 (13) |
| Prescription opioid without a prescriptiona (eg, taking someone else’s opioid pain medication) | 289 (27) |
| Legally prescribed nonopioid (eg, benzodiazapene) | 321 (8) |
| Prescription nonopioid without a prescriptiona (eg, taking someone else’s benzodiazepine) | 120 (11) |
| Nonprescription substance excluding heroin (eg, cocaine or methamphetamine) | 881 (22) |
| Heroina | 40 (4) |
| Other | 30 (1) |
a Reports 2016 data only, when TNSS surveillance added these variables. The denominator for percentages in these rows is 1063.
b Categories are not mutually exclusive.
Discussion
NAS is a consequence of the opioid crisis. Understanding the true incidence of NAS and the risk factors associated with the diagnosis is important. Since 2013, TNSS has provided data for understanding and responding to NAS. However, we demonstrated by using capture-recapture methods that both TNSS and HDDS underreported the number of infants with NAS in Tennessee by 48% and 32%, respectively. To our knowledge, our study is the first to use capture-recapture methods to estimate true rates of NAS in a statewide population.
Conversations with hospital staff members during a routine surveillance evaluation provided insights into possible reasons for underreporting infants with NAS (unpublished evaluations by telephone with a convenience sample of hospital staff members responsible for NAS surveillance, October 2018). Some staff members were unaware of Tennessee’s NAS reporting requirements. Additionally, anecdotal evidence from these conversations suggests provider bias toward diagnosing more severe cases. Although most providers used scoring rubrics and additional tests or maternal history to aid their NAS diagnoses, some acknowledged reluctance to give infants with mild symptoms an NAS diagnosis because of stigma. Most cases reported to TNSS in 2016 were classified as severe NAS, which may reflect a bias toward diagnosing and reporting infants with more severe withdrawal symptoms.
HDDS underestimation may be due to limitations inherent in using administrative data for public health purposes (eg, differences in billing and coding practices) and the same biases in reporting to TNSS. Determining the proportion of underreporting attributable to stigma, diagnosing bias, and other causes will require further evaluation. The Tennessee Department of Health can address some of these potential contributing factors by educating hospital staff members and providers about guidelines for diagnosing and reporting NAS.
Both TNSS and HDDS data are useful for identifying demographic, temporal, and geographic trends in NAS. Sex distribution in both data sets was consistent with previous studies showing male infants are more likely than female infants to be diagnosed with NAS and more likely to require pharmacotherapy.29 Geographic trends identified by both data sets are consistent with the geographic distribution of opioid prescriptions and areas with high use of MAT services. The counties with high rates of NAS were also identified as some of Tennessee’s most vulnerable counties in an assessment of HIV and hepatitis C risk.30 Only TNSS provided data on the sources of exposure for infants with NAS. HDDS alone would not, for example, have allowed identifying that approximately half of infants with NAS were born to women who were receiving MAT for substance use disorder.
Limitations
This study had 2 limitations. One limitation was that matching between data sets was limited by the lack of identifying variables shared by both data sets. A second limitation was that diagnosing and reporting NAS may be biased toward more severe cases.
Conclusion
Using data from 2 databases and capture-recapture methods demonstrated that the total burden of NAS in Tennessee was substantially higher than that identified in either database alone. Only TNSS captures data on the severity of the infant’s NAS and the mother’s exposure to drugs associated with NAS, highlighting its usefulness for guiding substance-specific interventions and policies. These results are important for public health departments that conduct or are considering conducting NAS surveillance, intervention planning, and policy development.
Acknowledgments
The authors thank Stacey Bosch, DVM, MPH (Epidemic Intelligence Service, Division of Scientific Education and Professional Development, CDC) and Nerissa Harvey, BA; Angela M. Miller, PhD, MSPH; Jane Yackley, MPH; and Julie Schaffner, MS, MPH (Tennessee Department of Health) for contributing to and supporting the writing of this article.
Authors’ Note: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Julia Brennan, MS, MPH  https://orcid.org/0000-0003-4719-4888
https://orcid.org/0000-0003-4719-4888
References
- 1. Ko JY, Patrick SW, Tong VT, Patel R, Lind JN, Barfield WD. Incidence of neonatal abstinence syndrome—28 states, 1999-2013. MMWR Morb Mortal Wkly Rep. 2016;65(31):799–802. doi:10.15585/mmwr.mm6531a2 [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Opioid overdose: US state prescribing rates, 2016. https://www.cdc.gov/drugoverdose/maps/rxstate2016.html. Published 2017. Accessed May 30, 2018.
- 3. Warren MD, Miller AM, Traylor J, Bauer A, Patrick SW. Implementation of a statewide surveillance system for neonatal abstinence syndrome—Tennessee, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(5):125–128. [PMC free article] [PubMed] [Google Scholar]
- 4. Gibbons CL, Mangen MJJ, Plass D. et al. Measuring underreporting and under-ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health. 2014;14:147 doi:10.1186/1471-2458-14-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jilani SM, Frey MT, Pepin D. et al. Evaluation of state-mandated reporting of neonatal abstinence syndrome—six states, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019;68(1):6–10. doi:10.15585/mmwr.mm6801a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Umer A, Loudin S, Maxwell S. et al. Capturing the statewide incidence of neonatal abstinence syndrome in real time: the West Virginia experience. Pediatr Res. 2019;85(5):607–611. doi:10.1038/s41390-018-0172-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lind JN, Ailes EC, Alter CC. et al. Leveraging existing birth defects surveillance infrastructure to build neonatal abstinence syndrome surveillance systems—Illinois, New Mexico, and Vermont, 2015-2016. MMWR Morb Mortal Wkly Rep. 2019;68(7):177–180. doi:10.15585/mmwr.mm6807a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Erwin PC, Lindley L, Meschke LL, Ehrlich SF. Neonatal abstinence syndrome in east Tennessee: characteristics and risk factors among mothers and infants in one area of Appalachia. J Health Care Poor Underserved. 2017;28(4):1393–1408. doi:10.1353/hpu.2017.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oei JL, Melhuish E, Uebel H. et al. Neonatal abstinence syndrome and high school performance. Pediatrics. 2017;139(2):e20162651 doi:10.1542/peds.2016-2651 [DOI] [PubMed] [Google Scholar]
- 10. Fill MA, Miller AM, Wilkinson RH. et al. Educational disabilities among children born with neonatal abstinence syndrome. Pediatrics. 2018;142(3):e20180 562. doi:10.1542/peds.2018-0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hunt RW, Tzioumi D, Collins E, Jeffery HE. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum Dev. 2008;84(1):29–35. doi:10.1016/j.earlhumdev.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 12. Maguire DJ, Taylor S, Armstrong K. et al. Long-term outcomes of infants with neonatal abstinence syndrome. Neonatal Netw. 2016;35(5):277–286. doi:10.1891/0730-0832.35.5.277 [DOI] [PubMed] [Google Scholar]
- 13. Kaltenbach K, O’Grady KE, Heil SH. et al. Prenatal exposure to methadone or buprenorphine: early childhood developmental outcomes. Drug Alcohol Depend. 2018;185:40–49. doi:10.1016/j.drugalcdep.2017.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones HE, Kaltenbach K, Heil SH. et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320–2331. doi:10.1056/NEJMoa1005359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Committee on Obstetric Practice. Committee opinion number 711: opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81–e94. doi:10.1097/AOG.00000000000002235 [DOI] [PubMed] [Google Scholar]
- 16. Nguyen L, Lander LR, O’Grady KE. et al. Brief report: treating women with opioid use disorder during pregnancy in Appalachia: initial neonatal outcomes following buprenorphine + naloxone exposure. Am J Addict. 2018;27(2):92–96. doi:10.1111/ajad.12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas CP, Fullerton CA, Kim M. et al. Medication-assisted treatment with buprenorphine: assessing the evidence. Psychiatr Serv. 2014;65(2):158–170. doi:10.1176/appi.ps.201300256 [DOI] [PubMed] [Google Scholar]
- 18. Centers for Medicare & Medicaid Services. ICD-9-CM diagnosis and procedure codes: abbreviated and full code titles. https://www.cms.gov/Medicare/Coding/ICD9ProviderDiagnosticCodes/codes.html . Updated May 20, 2014. Accessed July 20, 2017. [PubMed]
- 19. Centers for Medicare & Medicaid Services. 2018. ICD-10-CM and GEMs. https://www.cms.gov/Medicare/Coding/ICD10/2018-ICD-10-CM-and-GEMs.html . Updated August 11, 2017. Accessed July 20, 2017. [PubMed]
- 20. Boden LI. Capture-recapture estimates of the undercount of workplace injuries and illnesses: sensitivity analysis. Am J Ind Med. 2014;57(10):1090–1099. doi:10.1002/ajim.22247 [DOI] [PubMed] [Google Scholar]
- 21. McCarty DJ, Tull ES, Moy CS, Kwoh CK, Laporte RE. Ascertainment corrected rates: application of capture-recapture methods. Int J Epidemiol. 1993;22(3):559–565. doi:10.1093/ije/22.3.559 [DOI] [PubMed] [Google Scholar]
- 22. US Census Bureau. Annual estimates of the resident population: April 1, 2010, to July 1, 2016. 2016 population estimates. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk. Published June 2017. Accessed September 15, 2017.
- 23. Tennessee Department of Health, Division of Policy, Planning and Assessment. Report of Tennessee births. 2013. https://www.tn.gov/content/dam/tn/health/documents/TNBirths13.pdf. Published 2014. Accessed September 15, 2017.
- 24. Tennessee Department of Health, Division of Policy, Planning and Assessment. Number of live births with rates per 1,000 population, by race of mother, for counties of Tennessee, resident data, 2014. https://www.tn.gov/content/dam/tn/health/documents/TN_Births_-_2014.pdf. Accessed September 15, 2017.
- 25. Tennessee Department of Health, Division of Policy, Planning and Assessment. Number of live births with rates per 1,000 population, by race of mother, for counties of Tennessee, resident data, 2015. https://www.tn.gov/content/dam/tn/health/documents/TN_Births_-_2015.pdf. Accessed September 15, 2017.
- 26. Tennessee Department of Health, Division of Policy, Planning and Assessment. Number of live births with rates per 1,000 population, by race of mother, for counties of Tennessee, resident data, 2016. https://www.tn.gov/content/dam/tn/health/documents/TN_Births_-_2016.pdf. Accessed September 15, 2017.
- 27. SAS Institute, Inc. SAS Version 9.4. Cary, NC: SAS Institute, Inc; 2014. [Google Scholar]
- 28. ESRI. ArcMap Version 10.3. Redlands, CA: ESRI; 2010. [Google Scholar]
- 29. Charles KM, Cooper WO, Jansson LM, Dudley J, Slaughter JC, Patrick SW. Male sex associated with increased risk of neonatal abstinence syndrome. Hosp Pediatr. 2017;7(6):328–334. doi:10.1542/hpeds.2016-0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rickles M, Rebeiro PF, Sizemore L. et al. Tennessee’s in-state vulnerability assessment for a “rapid dissemination of human immunodeficiency virus or hepatitis C virus infection” event utilizing data about the opioid epidemic. Clin Infect Dis. 2018;66(11):1722–1732. doi:10.1093/cid/cix1079 [DOI] [PMC free article] [PubMed] [Google Scholar]