Figure 1.
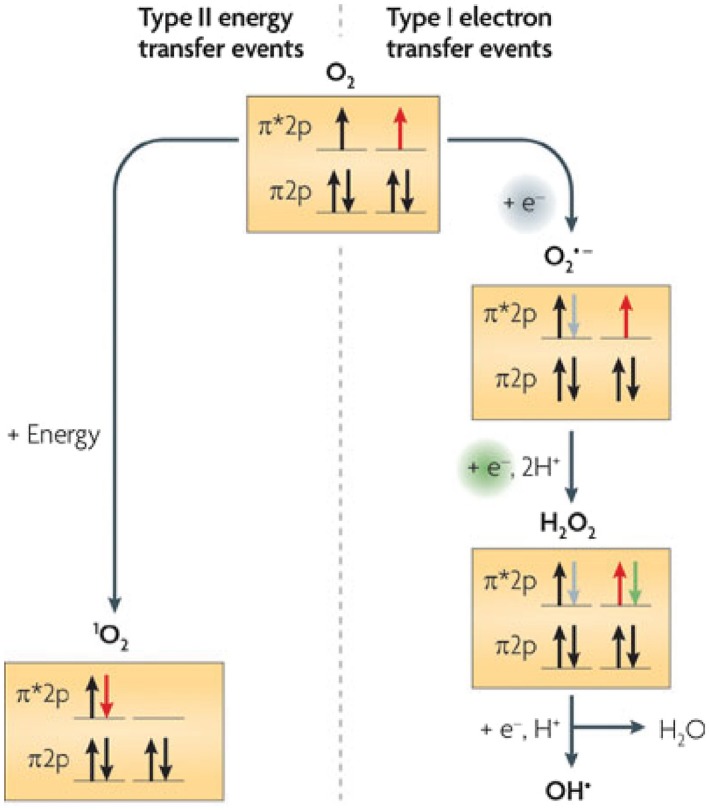
Formation and biological consequences of ROS generation. The right panel shows production of the ROS superoxide (O2 •−), hydrogen peroxide (H2O2) or hydroxyl radicals (OH•) by one‐electron transfer reactions. The left panel shows formation of singlet oxygen (1O2) by Type II energy transfer, typically from an excited, triplet state donor, to O2. The diagrams show the spin of electrons in shells of the outer p orbital of each compound. Note that 1O2 is formed by movement of an electron between outer p orbital shells (red arrow). Figure modified from (Ziegelhoffer and Donohue, 2009).
