Figure 3.
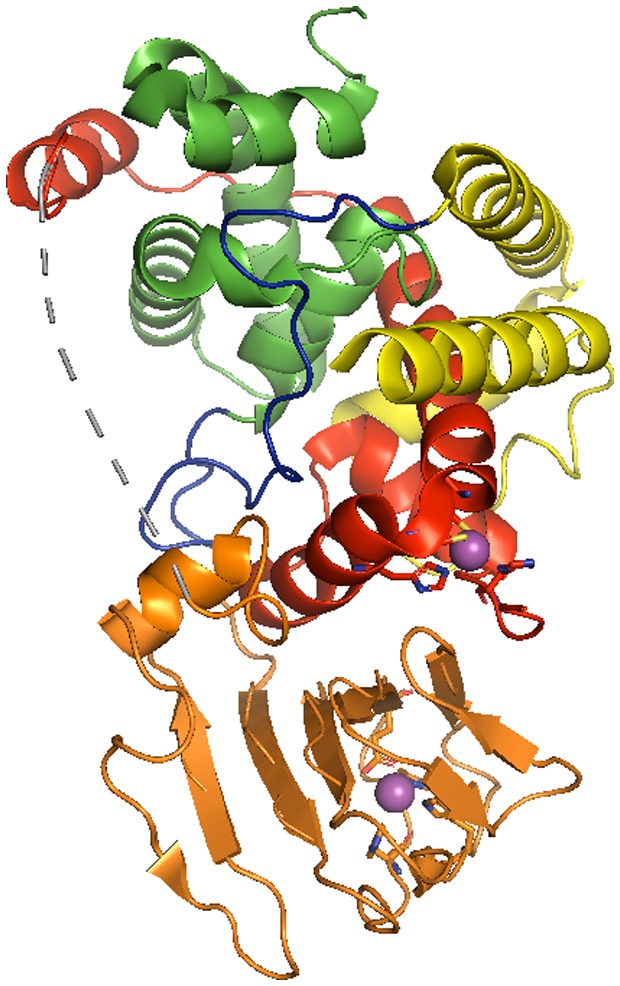
Structure of the Rb. sphaeroides σE‐ChrR complex. The Rb. sphaeroides σE protein is colored to reflect the major functional domains of sigma factors (green‐region 2; blue‐region 2‐4 linker; yellow‐region 4). ChrR is colored to indicate its two major structural domains (red‐the 4 helical bundle that contains the N‐terminal anti‐sigma domain, ASD; orange‐the C‐terminal cupin‐like domain, CLD). Note the extensive interactions of the ChrR‐ASD with σE regions 2 and 4. The 2 zinc atoms associated with ChrR are shown in magenta, along with the side chains of the amino acids that ligate these metals (6His, 31His, 35Cys & 38Cys in the ChrR‐ASD and 141His, 143His, 147Glu and 177His in the ChrR‐CLD respectively).
