Figure 4.
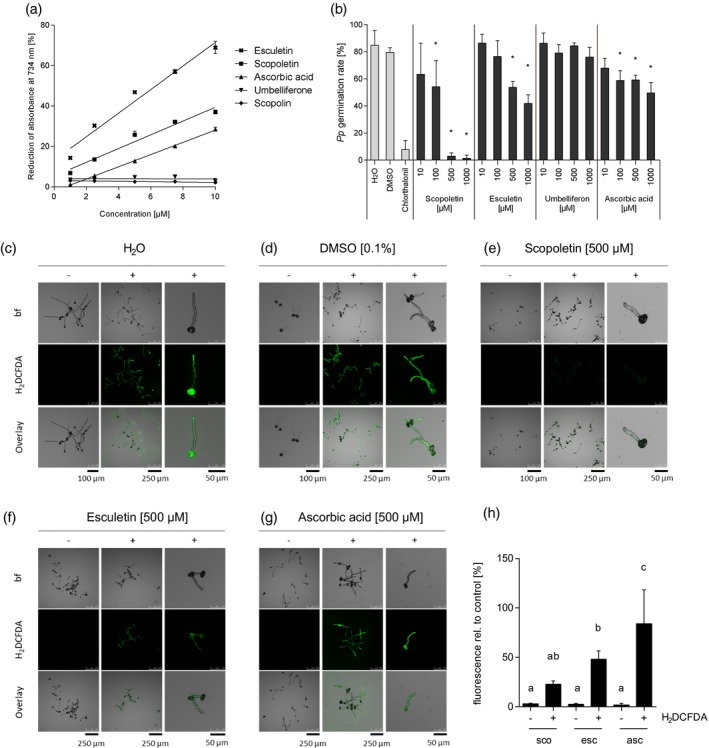
Suppression of Pp germination correlates with reduced fungal ROS accumulation in pre‐infection structures.
(a) Esculetin ( ) and scopoletin (
) and scopoletin ( ) but not scopolin (
) but not scopolin ( ) or umbelliferone (
) or umbelliferone ( ) have ROS‐scavenging activity. Antioxidant capacity of the coumarins was determined in an ABTS assay and compared with ascorbic acid (
) have ROS‐scavenging activity. Antioxidant capacity of the coumarins was determined in an ABTS assay and compared with ascorbic acid ( ) as an antioxidant reference. Reduction of ABTS∙+ radicals by antioxidants correlates with a reduction of absorbance at 734 nm. Shown are mean values and SD from three independent experiments.
) as an antioxidant reference. Reduction of ABTS∙+ radicals by antioxidants correlates with a reduction of absorbance at 734 nm. Shown are mean values and SD from three independent experiments.
(b) Non‐antioxidant umbelliferone is incapable of inhibiting Pp germination whereas ROS scavengers esculetin and ascorbic acid inhibit Pp development. However, they are far less fungistatic than scopoletin. Germination rate of at least 100 Pp uredospores was determined microscopically 16 h after incubation on polyethylene foil in the presence of different coumarins or ascorbic acid at different concentrations. Water was used as a negative control for ascorbic acid treatment and 0.1% DMSO served as negative control for treatments with scopoletin, esculetin and umbelliferone, all coumarin samples containing 0.1% DMSO. The contact fungicide chlorothalonil (15 μg ml−1 in 0.01% DMSO) was included as a known inhibitor of Pp germination. Average values and SD from three independent experiments are shown. Asterisks indicate significant differences to the appropriate negative control (Holm–Sidak's multiple comparisons test; P < 0.05). Histochemical H2DCFDA staining discloses ROS production in germinated Pp uredospores and germ tubes and intracellular ROS‐scavenging capacity of different coumarins and ascorbic acid. Spores were incubated in water for 3 h to allow for germination and then supplemented with water (c), 0.1% DMSO (d) or 500 μm of each, scopoletin (e), esculetin (f) or ascorbic acid (g). After 1 h, H2DCFDA was added to a final concentration of 2.5 μg ml−1 and incubated for 30 min. ROS accumulation was analyzed by monitoring the fluorescence intensity upon H2DCFDA staining in a confocal laser scanning microscope (488 nm excitation and 500–550 nm emission).
(h) For semi‐quantitative determination of fungal ROS accumulation maximum H2DCFDA fluorescence was determined in >100 Pp germ tubes in at least three independent experiments. Shown is the average maximum H2DCFDA fluorescence in treated germ tubes relative to the respective control. Groups a, b and c are significantly different from each other in one‐way analysis of variance (anova) (Holm–Sidak method, P < 0.05).
