Abstract
White matter (WM) integrity has been related to cognitive ability in adults and children, but it remains largely unknown how WM maturation in early life supports emergent cognition. The associations between tract‐based measures of fractional anisotropy (FA) and axial and radial diffusivity (AD, RD) shortly after birth, at age 1, and at age 2 and cognitive measures at 1 and 2 years were investigated in 447 healthy infants. We found that generally higher FA and lower AD and RD across many WM tracts in the first year of life were associated with better performance on measures of general cognitive ability, motor, language, and visual reception skills at ages 1 and 2, suggesting an important role for the overall organization, myelination, and microstructural properties of fiber pathways in emergent cognition. RD in particular was consistently related to ability, and protracted development of RD from ages 1 to 2 years in several tracts was associated with higher cognitive scores and better language performance, suggesting prolonged plasticity may confer cognitive benefits during the second year of life. However, we also found that cognition at age 2 was weakly associated with WM properties across infancy in comparison to child and demographic factors including gestational age and maternal education. Our findings suggest that early postnatal WM integrity across the brain is important for infant cognition, though its role in cognitive development should be considered alongside child and demographic factors.
Keywords: brain, cognition, diffusion tensor imaging, infancy, neurodevelopment, tractography
1. INTRODUCTION
The first 2 years of life mark an accelerated, dynamic period of postnatal human brain development. During this time, the brain more than doubles in size (Knickmeyer et al., 2008), the cortex expands rapidly (Li et al., 2014; Lyall et al., 2015), and the rate of brain white matter (WM) myelination peaks (Dubois, Dehaene‐Lambertz, Kulikova, & Poupon, 2014). The rapid development of postnatal WM has been well studied in vivo using diffusion tensor imaging (DTI), a technique that probes the diffusivity of water molecules in the brain. In WM, diffusion anisotropy, commonly measured by fractional anisotropy (FA), is high, while isotropic diffusion, measured by axial and radial diffusivity (AD and RD), is low relative to gray matter and unmyelinated WM (Dubois et al., 2014). In the first 2 years of life, these metrics change rapidly as fibers are organized into bundles, premyelination is initiated, and myelination occurs; FA increases, while AD and RD decrease (Dubois et al., 2014; Geng et al., 2012). Postmortem studies have shown that myelination occurs in proximal before distal pathways, in central sites before poles, and in occipital poles before frontotemporal poles (Brody, Kinney, Kloman, & Gilles, 1987; Kinney, Brody, Kloman, & Gilles, 1988), and neuroimaging studies of WM development report similar findings (Deoni et al., 2011; Deoni, Dean III, O'Muircheartaigh, Dirks, & Jerskey, 2012; Gao et al., 2009; Geng et al., 2012). During this same period, the cognitive capacities of infants advance from that of basic functions to complex tasks including the refinement of fine and gross motor skills, processing of visual cues, and the comprehension and production of language. While it appears that the sequence of myelination mirrors that of cognitive development, given that myelination occurs in primary sensory tracts before motor tracts and in projection pathways before higher‐order association pathways (Guillery, 2005), it remains largely unknown as to how WM matures to support cognition (Walhovd, Tamnes, & Fjell, 2014).
It is hypothesized that one potential mechanism by which the brain mediates cognition is through optimized, rapid information transfer between connected cortical and subcortical regions via myelinated axons (Mabbott, Noseworthy, Bouffet, Laughlin, & Rockel, 2006; Nagy, Westerberg, & Klingberg, 2004). Axons wrapped in myelin sheaths conduct action potentials and neuronal signals at much faster rates due to the insulative properties of myelin (Dubois et al., 2014). Additionally, it has been shown that myelination can occur in an activity‐dependent manner, such that oligodendrocytes appear to selectively myelinate axons which receive more input from neurons (Fields, 2015; Wake et al., 2015), thus myelination likely plays a crucial role in shaping structural connectivity, communication between brain regions, and ultimately learning and cognition. Given that the sequential development of myelination in the brain mirrors cognitive development, myelination may be a prerequisite for cognitive development in early life. Additionally, neural activity that occurs during learning may accelerate myelination and increase WM integrity, a process that could be particularly prominent during early life when learning occurs rapidly.
There is significant evidence to support a link between cognition and WM microstructure, measured using DTI in adults (Zatorre, Fields, & Johansen‐Berg, 2012), including findings correlating the integrity of major WM fiber bundles with information processing speed in healthy older adults (Penke et al., 2010), language learning in young adults (Mamiya, Richards, Coe, Eichler, & Kuhl, 2016), and improvements in working memory through training (Takeuchi et al., 2010). Recent studies have begun to demonstrate links between early WM development and cognition in healthy infants and toddlers. WM integrity of fiber tracts supporting working memory in adults has been related to visuospatial working memory performance in 1‐year‐olds (Short et al., 2013), trajectories of myelin water fraction across the first 5 years of life have been related to cognitive ability both globally and regionally (Deoni et al., 2014; O'Muircheartaigh et al., 2013), and common factors of DTI parameters from twelve major fiber bundles have been related to cognitive performance across the first 2 years of life (Lee et al., 2017). Taken together, these studies suggest that both global and local WM development may be reflective of cognitive development across early childhood. However, these studies have limitations which include the averaging of cognitive scores across development (Deoni et al., 2014), only investigating one cross‐sectional time point (Short et al., 2013), and using only tracts thought to support cognition in aging adults (Lee et al., 2017). Finally, none of these studies have provided an in‐depth description of how individual WM tract maturation relates to both general ability and domain‐specific cognition in the first 2 years of life.
In this study, our goal was to extend the work in the field by describing the relationships between tract‐level maturation and emerging cognition in infancy and toddlerhood. Specifically, we sought to determine the relationship of tract‐based measures of FA, AD, and RD derived from neonatal, 1‐year, and 2‐year DTIs to cognitive measures of general ability, language, motor, and visual reception skills at ages 1 and 2 years in a sample of 447 healthy children. This extends our previous study which found that common factors of FA, AD, and RD across major tracts were related to infant and toddler ability (Lee et al., 2017) by exploring a fundamental question: are associations between WM and cognition global in nature or are they attributable to individual differences in the microstructural integrity of certain tracts? Additionally, we studied how WM microstructure and cognitive development are influenced by major child‐level and sociodemographic factors like gestational age and maternal education level. We also explored how WM integrity may mediate associations between these individual difference factors and cognition. This information will provide important insight into how measures of WM microstructure perform as biomarkers of present and future cognitive ability and provide important context for interpreting associations between WM development and emerging cognition in infancy and toddlerhood. We hypothesized that tract‐based measures of WM integrity would be related to present and future cognitive ability, with more mature properties (higher FA, lower AD, and RD) relating to better performance on measures of general cognitive ability, motor, language, and visual reception skills. Given that previous findings implicate both specific tracts and global WM in emerging cognition, we hypothesized that ability may be related to WM microstructure in many tracts, but that these WM‐cognition associations may become more anatomically specific as children develop. We also explored how trajectories of maturation across the first 2 years of life in these WM tracts related to cognitive ability at age 2, which, to the best of our knowledge, has not been done on a tract‐by‐tract basis.
2. MATERIALS AND METHODS
2.1. Participants
Participants were part of the UNC Early Brain Development Study, an ongoing study of human brain development in singletons and twins (Gilmore et al., 2010; Knickmeyer et al., 2008, 2016). Pregnant women were recruited from outpatient obstetrics and gynecology clinics at the University of North Carolina Hospitals and Duke University Medical Center. Mothers were excluded from the study for a major illness or use of illegal drugs during pregnancy. Magnetic resonance images (MRIs) were collected shortly after birth, and at ages 1 and 2 years for all offspring participants. Cognitive assessments were also collected at 1‐ and 2‐year visits. From the total 1,330 infant participants recruited as part of this study, we retrospectively identified 447 participants with at least one diffusion weighted image (DWI) that produced usable quantitative tractography data (10% of entire sample excluded) and at least one cognitive assessment (32% excluded) who met the following inclusion criteria: no diagnosis of major psychiatric disorder in the mother (8% excluded), born at ≥32 weeks gestation (moderately premature to full term; 3% excluded), spent ≤24 hr in the neonatal intensive care unit following birth (9% excluded), and had no major abnormalities noted on any MRI or major medical issues or illnesses reported up to age 2 (4% excluded). We also excluded 2 infants (<1%) due to late follow up resulting in their first MRI scans being taken more than 100 days post birth. Table 1 outlines the demographic characteristics of the sample. Informed written consent and parental permission were obtained from at least one parent of all child participants and all study protocols were approved by the University of North Carolina at Chapel Hill's Institutional Review Board.
Table 1.
Participant demographics
| Child Characteristics | N/Mean (SD/Percent), Median [Min, Max] |
|---|---|
| Gestational age at birth (weeks) | 38.10 (1.77), 38.0 [35.29, 41] |
| Infants born preterm (<37 weeks) | 126 (28.19%) |
| Birth weight (g) | 2,984.3 (581.20), 2,974 [2,225, 4,659] |
| Stay in NICU | 19 (4.25%) |
| Age at Neo MRI (weeks) | 3.89 (2.15), 3.3 [1.71, 8.71] |
| Age at 1 year MRI (weeks) | 55.81 (3.56), 5.14 [51.14, 62.71] |
| Age at 2 year MRI (weeks) | 108.07 (4.47), 107.57 [99.0, 111.14] |
| Age at 1 year Mullen (weeks) | 55.51 (3.35), 55.0 [49.71, 62.71] |
| Age at 2 year Mullen (weeks) | 107.57 (3.80), 106.86 [99.0, 113.0] |
| 1 year ELC | 116.37 (12.88), 117 [78, 134] |
| 2 year ELC | 107.75 (15.51), 110 [71, 136] |
| Male | 240 (53.69%) |
| Female | 207 (46.31%) |
| Single gestation | 211 (47.20%) |
| Twin gestation | 236 (52.80%) |
| Zygosity | |
| Dizygotic twins | 137 (58.80%) |
| Monozygotic twins | 85 (36.48%) |
| Opposite sex twins | 11 (4.72%) |
| Parental characteristics a | |
|---|---|
| Maternal age (years) | 30.23 (5.47), 30 [18, 40] |
| Paternal age (years) | 32.11 (5.96), 32 [19, 42] |
| Mother education (years) | 15.61 (3.37), 16 [8, 25] |
| Father education (years) | 15.19 (3.68), 16 [2, 26] |
| Total household income ($) | $74,128 ($55, 575), $68,000 [$3,120, $150,000] |
| Maternal/paternal race | |
| White | 339 (75.84%)/319 (72.50%) |
| American Indian or Alaskan Native | 2 (0.45%)/0 (0%) |
| African American | 95 (21.25%)/103 (23.41%) |
| Asian | 11 (2.46%)/18 (4.09%) |
| Maternal/paternal ethnicity | |
| Hispanic | 50 (11.19%)/58 (13.12%) |
| Non‐Hispanic | 397 (88.81%)/384 (86.88%) |
Reported at the time of the child's birth
2.2. Image acquisition
DWI data were acquired between 2004 and 2014 using a single‐shot echo‐planar imaging spin‐echo sequence at 3 T on either a Siemens Allegra head‐only or a Siemens Tim Trio scanner (Siemens Medical System, Inc., Erlangen, Germany), which replaced the Allegra in 2011. For earlier collected Allegra DWI data, a 6‐direction protocol was used with the following parameters: Repetition Time (TR)/ Echo Time (TE) = 5,200/73 ms, slice thickness = 2 mm, and in‐plane resolution = 2 × 2 mm2, with a total of 45 slices in 6 unique directions using b value of 1,000 s/mm2 and 1 baseline image (b value = 0) per sequence. This sequence was repeated five times (generating a total of 35 DWIs) to improve signal‐to‐noise. For the remaining Allegra DWI data, 42 directions of diffusion sensitization were acquired with a b value of 1,000 s/mm2 in addition to seven baseline (b value = 0) images (generating a total of 49 DWIs). The parameters for the 42‐direction data were as follows: TR/TE/Flip angle = 7,680/82/90°, slice thickness = 2 mm, and in‐plane resolution = 2 × 2 mm2, with a total of 60–72 slices. The rest of the study subjects scanned on the Tim Trio followed the same sequencing parameters as the 42‐direction Allegra protocol detailed above. For information on the number of scans collected with each scanner and protocol (Allegra 6‐direction [A6], Allegra 42‐direction [A42], and Trio 42‐direction [T42]; Table 2).
Table 2.
Diffusion tensor imaging acquisition protocols
| Scanner and direction | Neonate | Year 1 | Year 2 | Total scans |
|---|---|---|---|---|
| Allegra, 6 direction (A6) | 183 (55.12%) | 87 (34.12%) | 61 (33.70%) | 331 (43.1%) |
| Allegra, 42 direction (A42) | 100 (30.12%) | 119 (46.67%) | 78 (43.09%) | 297 (38.67%) |
| Trio, 42 direction (T42) | 49 (14.76%) | 49 (19.22%) | 42 (23.30%) | 140 (18.23%) |
Scan frequencies are listed by year and scanner and direction protocol. Percentages for each scanner and direction combination are listed by year and for all scans.
2.3. DTI processing and quantitative tractography
A study‐specific, automated quality control (QC) protocol was applied to all raw DWI data using DTIPrep (http://www.nitrc.org/projects/dtiprep) which detected slice‐wise and gradient‐wise intensity and motion artifacts and corrected for motion and eddy current effects (Oguz et al., 2014). Diffusion images with large motion artifacts and missing or corrupted gradients were excluded from further processing. On average, 7.88 (standard deviation [SD] = 3.31), 5.43 (SD = 3.79), and 5.56 (SD = 4.19) gradients were automatically excluded from neonatal, 1‐year, and 2‐year 6‐direction images, and 4.03 (SD = 4.53), 2.94 (SD = 2.69), and 2.37 (SD = 2.90) from 42‐direction images respectively. Additionally, an average of 0.79 (SD = 1.57), 1.45 (SD = 1.92), and 1.21 (SD = 1.76) gradients were excluded from 6‐direction and 0.56 (SD = 1.30), 1.63 (SD = 2.44), and 1.85 (SD = 2.69) gradients were excluded from 42‐direction images from neonatal, 1‐year, and 2‐year scans, respectively, upon visual inspection by an experienced rater. Overall, neonatal, 1‐year, and 2‐year images analyzed in this study contained an average of 31.20 (SD = 6.82), 34.25 (SD = 2.69), and 34.56 (SD = 5.86) gradients, respectively.
Skull and nonbrain tissue were removed using the Brain Extraction Tool (Smith, 2002), and tensors were estimated using a weighted least‐squares algorithm (Goodlett, Fletcher, Gilmore, & Gerig, 2009). The neonatal and pediatric (1‐ and 2‐year) DTI atlases (https://www.nitrc.org/projects/uncebds_neodti) were created using the UNC‐UTAH NAMIC DTI framework (http://www.nitrc.org/projects/dtiatlasbuilder) outlined by Verde et al. (2014). A total of 45 homologous tracts were defined in both atlases using streamline deterministic tractography in 3D Slicer (http://www.slicer.org). Our analyses focused on 13 bilateral and 3 commissural tracts chosen for their canonical involvement in higher‐order cognition (cingulum, superior longitudinal fasciculus, inferior fronto‐occipital fasciculus, inferior longitudinal fasciculus, uncinate, genu, rostrum, and splenium), sensory‐motor relay (corticothalamic projections, corticofugal motor projection), and language (frontoparietal, frontotemporal, and temporoparietal segments of the arcuate), which are all domains that are captured in the infant and toddler cognitive assessments. Tracts used in our analyses, along tract name abbreviations, can be seen in Figure 1; for additional details on tractography, see the appendix from Lee et al. (2015). Nonlinear, diffeomorphic pair‐wise registration was performed to map individual subject DTIs into atlas space, and registration accuracy was visually inspected in DTI‐AtlasBuilder (http://www.nitrc.org/projects/dtiatlasbuilder) to determine if the computed transforms were appropriate. Resulting deformation fields were then used to map atlas fibers, which were tracked in atlas space, into individual subject space, where profiles of FA, AD, and RD were extracted at evenly spaced points (arc lengths) along each fiber tract (DTI‐Reg, DTIAtlasFiberAnalyzer; http://www.slicer.org). Each tract from each subject underwent QC using FADTTSter (http://www.nitrc.org/projects/fadttster) prior to statistical analysis and subjects were excluded on a tract‐by‐tract basis if their correlation with the population average FA profile was <0.70. Some tracts were less reliably reproduced in individuals and had higher failure rates than others, and terminal arc lengths from some tracts were not included in tract‐average calculations due to high noise. Average FA, AD, and RD values were computed for each tract. For details on tract‐level QC, see Supporting Information.
Figure 1.
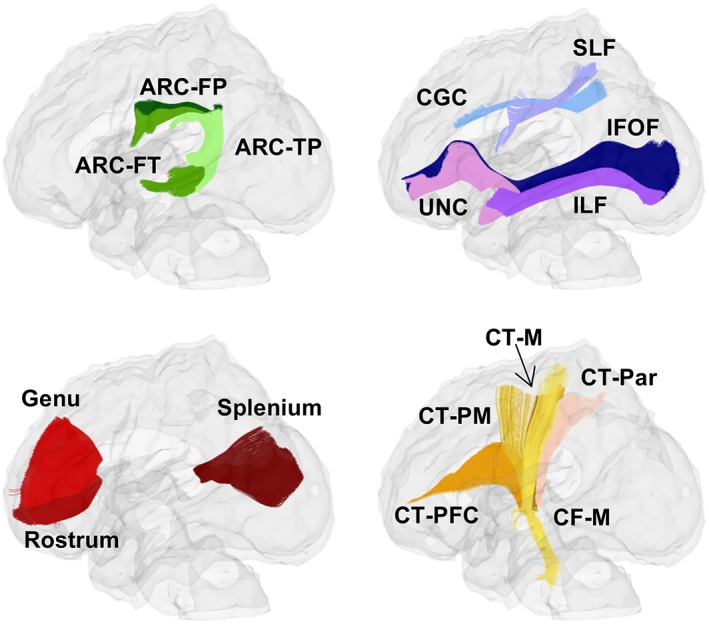
Neonatal and pediatric white matter tracts. Tracts analyzed in the study are displayed. Abbreviations for tracts are as follows: Arcuate frontoparietal (ARC‐FP), frontotemporal (ARC‐FT), and temporoparietal (ARC‐TP) segments, cingulum (CGC), superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus (ILF), inferior fronto‐occipital fasciculus (IFOF), uncinate (UNC), genu, rostrum, splenium, corticofugal motor tracts (CF‐M; yellow, partially transparent to show parallel CT‐M), and the corticothalamic prefrontal (CT‐PFC), premotor (CT‐PM), motor (CT‐M; brown, indicated with a black arrow), and parietal (CT‐Par) tracts. To denote left or right, “(L)” or “(R)” are added to tract abbreviations in tables [Color figure can be viewed at http://wileyonlinelibrary.com]
2.4. Cognitive assessments
Cognitive ability was assessed at ages 1 and 2 using the Mullen Scales of Early Learning (MSEL). Child measures of gross motor (GM), fine motor (FM), visual reception (VR), expressive and receptive language (EL and RL) were collected by experienced testers. Performance on the latter four MSEL cognitive scales was analyzed as raw scores, and their age‐standardized t‐scores were combined into an early learning composite (ELC) standardized score (range: 49–155, mean = 100, SD = 15). The ELC has high internal consistency (median = 0.91) and reliability (median = 0.84 for the cognitive scales during these testing ages), and principal factor loadings of the scales lend support for the construct validity of the ELC as a general measure of cognitive ability (Mullen, 1995), much like an intelligence quotient. The primary measure of interest for this study was the ELC, though we also investigated MSEL raw scale scores (not normalized for age range) for each of the four cognitive domains. We specifically chose to study raw domain‐specific scores because we are interested in understanding how a child's actual performance relates to brain development, instead of attempting to interpret the relationships between brain development and a child's degree of difference from a normative sample, a rationale which has been previously described (Naigles et al., 2017). A subset of the MSEL assessments (5 and 7% of MSEL tests at ages 1 and 2, respectively) were conducted in Spanish to match the native language of the child.
2.5. Statistical analysis
The analytic approach for this project was conducted in five major steps. First, we established the presence and strength of associations between WM tract integrity shortly after birth, at age 1, and at age 2 and emerging cognition by calculating within‐subject Pearson's correlations that were not adjusted for covariates. All possible cross‐sectional and longitudinal relationships were assessed: (a) neonatal WM correlating with MSEL scores at ages 1 and 2, (b) WM at age 1 correlating with MSEL scores at age 1 and 2, and (c) WM at age 2 correlating with MSEL scores at age 2. The goal for this step was two‐fold: first, we wanted to ensure associations existed between WM and cognition in this age group that could be further probed, and, second, we intended to use these associations—which were not adjusted for covariates—as a baseline for comparing results generated in later steps after adjustment for covariates. Having this baseline for comparison allows us to explore how infant WM serves as a biomarker of emergent cognition relative to child and demographic factors.
Second, we sought to identify child and demographic factors that were associated with both WM tract integrity and cognition in this age range to use as covariates in our models moving forward. In an overlapping dataset, we have shown that gestational age at birth, age at cognitive testing, gestational number (twin versus singleton), sex, and maternal education level, and a nuisance variable controlling for the time elapsed since the start of MSEL data collection at each 1‐ and 2‐year study visit (MSEL test date), are related to infant cognitive scores (Girault et al., 2018). We used mixed model analysis to confirm these findings in the current dataset. To describe the nature of the cognitive developmental assessments used in this study, we also computed correlations between cognitive scores over time for the ELC and domain‐specific scores. Next, we explored the development of DTI parameters over time in this sample and probed relationships between child and demographic factors and DTI parameters by tract. We plotted longitudinal trajectories of parameters by tract and calculated Pearson's correlations between AD, RD, and FA over time to establish developmental trends. We calculated correlations between DTI parameters across tracts to establish the interrelatedness of tract development (i.e., are certain groups of tracts more closely correlated in terms of maturation level). We also calculated Pearson's correlations to assess the strength of associations between DTI parameters at each age (i.e., neonatal AD vs. AD at age 1) and between DTI parameters at the same age (i.e., neonatal AD vs. neonatal FA). Finally, we calculated and compared the average standard deviations across tracts shortly after birth, at age 1, and at age 2 to quantify inter‐subject variability in DTI metrics across development. To test for associations with demographic factors, we calculated Pearson's correlations between DTI parameters and primary measures of interest including maternal education level, gestational age at birth, and sex.
Third, we determined the relative associations between cognition and WM tract integrity during early postnatal development after adjusting Pearson's correlations for the covariates identified in the previous step (gestational age at birth, gestation number, age at MSEL testing, sex, maternal education level, plus nuisance variables including MSEL test date, and scanner and protocol variables). We then compared the unadjusted correlations from the first step to the adjusted correlations in this step, which allowed us to determine the usefulness of tract‐based WM integrity at these ages as biomarkers of infant and toddler cognitive abilities.
Fourth, a mediation analysis was performed to evaluate a hypothesis generated during the previous steps regarding WM tract integrity as a possible mediator of associations between gestational age at birth and general cognitive ability at age 2, given the strong association between gestational age and neonatal WM, associations found between gestational age and 2‐year ELC scores, and associations found between neonatal WM and 2‐year ELC scores. The mediation analysis involved four steps: (a) show that gestational age at birth and ELC scores at age 2 are correlated, (b) show that gestational age at birth is correlated with the mediator, neonatal FA, AD, and RD, treating the mediator as a response variable, (c) show that neonatal FA, AD, and RD affect ELC scores at age 2 while controlling for gestational age, and (d) use a Sobel test (M. E. Sobel, 1982) to evaluate the significance of the mediation. All steps were tested using linear regression models, with FA, AD, and RD for each tract tested separately.
Fifth, and finally, we performed a longitudinal analysis to determine if tract‐specific WM developmental trajectories in the first 2 years of life are associated with cognitive abilities at age 2. Specifically, we tested if tract‐based measures of neonatal WM (as a reflection of prenatal and very early postnatal brain development; FA0, AD0, and RD0), the change in WM properties in the first year of postnatal life (dFA1,0, dAD1,0, and dRD1,0; calculated as a simple subtraction of the parameter at the earlier age from that of the later age), or the change in WM properties in the second year of life (dFA2,1, dAD2,1, and dRD2,1) related to MSEL 2‐year scores, our latest testing point. To do this, we used linear mixed‐effects models predicting 2‐year scores including all three WM measures (i.e., FA0, dFA1,0, and dFA2,1) simultaneously while controlling for gestational age (GA), maternal education (MEDUY), age at MSEL testing (AgeMSEL), sex, and nuisance variables related to DTI protocol at each age (ScanDir) and MSEL test date (DATEMSEL). Only subjects with complete longitudinal data—neonatal, 1‐year, and 2‐year scans and cognitive data at age 2—were included in these analyses, and one twin from each pair was treated as a repeated measure. The statistical model for FA predicting ELC at age 2 (ELC2) is shown below:
where ELC2 is the dependent variable and FA0 , dFA1, 0 , dFA2, 1 , GA, AgeMSEL, sex, MEDUY, ScanDir0, ScanDir1, ScanDir2, and DATEMSEL are the independent variables, and ε is the random error. The models for AD and RD predicting each MSEL 2‐year score were constructed in the same manner.
We performed several sensitivity analyses. To test for possible differences in WM‐cognition associations between full‐term (≥37 weeks gestation) and preterm (<37 weeks) participants, we re‐ran primary analyses separately in each group. For the unadjusted Pearson's correlations between DTI parameters and MSEL scores which did not include gestation number as a covariate, we also split the sample into twins and singletons to test for the potential group differences in WM‐cognition associations. Finally, as a sensitivity check of our main findings from the adjusted Pearson's correlations between DTI parameters and cognitive scores we re‐ran primary analyses using mixed‐effects models. Mixed effects models allow us to account for the relatedness of twins in our sample by treating one twin from each pair as a repeated measure with compound symmetric covariance structure. Finally, we also computed partial Pearson's correlations correcting for age and sex only, as a means to understand how these covariates, especially age, may account for the presence of associations between cognitive development and WM development.
Sample sizes for all analyses are reported in Table 3. All results from DTI analyses are corrected for multiple comparisons using False Discovery Rate (Benjamini & Hochberg, 1995) at p < .05, such that each model predicting MSEL scores (i.e., neonatal WM correlating with ELC scores at age 1 being a single model) is corrected for the number of tracts analyzed per DTI parameter (i.e., FA is modeled independently, corrected for the inclusion of data from 29 tracts, treating the left and right tracts separately). All statistical analyses were performed using SAS statistical software, version 9.4.
Table 3.
Sample sizes across analyses
| Pearson's correlations | ||
|---|---|---|
| N (% of entire sample); [Min, Max] | ||
| Neo DTI–1 year MSEL | 307 (69%); [207, 317] | |
| Neo DTI–2 year MSEL | 251 (56%); [168, 260] | |
| 1 year DTI–1 year MSEL | 251 (56%); [232, 254] | |
| 1 year DTI–2 year MSEL | 193 (43%); [176, 195] | |
| 2 year DTI–2 year MSEL | 169 (38%); [160, 171] | |
| Mixed effects models | ||
|---|---|---|
| Unique subjects | Repeated measure | |
| N (%); [Min, Max] | N [Min, Max] | |
| Longitudinal | 57 (13%); [39, 59] | 7 [4, 8] |
3. RESULTS
3.1. Cognitive assessments: developmental patterns and influences of demographic factors
Results from mixed models testing for the association between cognitive scores and demographic factors in this sample are presented in Table 4. Factors influencing 1‐year MSEL scores were chronological age at testing, MSEL test date, gestational age at birth, and sex; all of which had very small impacts on MSEL scores at age 1. Sex differences were more pronounced, with males scoring more than two points lower than females on the ELC at age 1.
Table 4.
Mixed model summary results relating covariates to MSEL scores
| Age at Testing | Test Date | Maternal Education | Gestational Age at Birth | Gestation Number | Sex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | p | Estimate | p | Estimate | p | Estimate | p | Estimate | p | Estimate | p | ||
| YEAR 1 MSEL | ELC | −0.12 | .0004 | 4.7E‐03 | <.0001 | −0.26 | .2247 | −0.09 | .2416 | 1.21 | .5208 | −2.48 | .05 |
| GM | 0.04 | <.0001 | 7.3E‐04 | <.0001 | −0.07 | .1343 | 0.05 | .0050 | −0.32 | .442 | −0.47 | .0993 | |
| FM | 0.03 | <.0001 | 2.4E‐04 | .0123 | −0.02 | .5324 | 0.04 | .0002 | 0.37 | .1191 | 0.10 | .5304 | |
| EL | 0.02 | <.0001 | 7.4E‐04 | <.0001 | −0.08 | .0078 | 0.03 | .0195 | 0.12 | .65 | −0.38 | .0325 | |
| RL | 0.03 | <.0001 | 5.2E‐04 | <.0001 | −0.03 | .3675 | 0.03 | .0109 | −0.03 | .9114 | −0.48 | .0098 | |
| VR | 0.04 | <.0001 | 3.5E‐04 | .0044 | 0.04 | .1997 | 0.03 | .0119 | −0.04 | .9041 | −0.51 | .0158 | |
| YEAR 2 MSEL | ELC | −0.02 | .6338 | 8.5E‐04 | .3389 | 1.77 | <.0001 | 0.16 | .0879 | 5.60 | .0138 | −4.71 | .0032 |
| GM | 0.01 | .0707 | −2.2E‐04 | .0802 | 0.04 | .2582 | 0.01 | .4874 | 0.06 | .8539 | 0.04 | .846 | |
| FM | 0.02 | .0015 | 1.0E‐04 | .4308 | 0.11 | .0022 | 0.05 | .0005 | 0.40 | .2109 | −0.59 | .01 | |
| EL | 0.02 | .0034 | 3.5E‐04 | .1025 | 0.30 | <.0001 | 0.08 | .0003 | 0.76 | .1645 | −1.15 | .0025 | |
| RL | 0.01 | .1182 | 1.3E‐04 | .5157 | 0.35 | <.0001 | 0.03 | .1566 | 1.24 | .0116 | −0.58 | .093 | |
| VR | 0.01 | .0757 | 2.5E‐06 | .9909 | 0.36 | <.0001 | 0.02 | .4223 | 0.86 | .111 | −1.07 | .0057 | |
MSEL scores at age 2 were most consistently related to maternal education, with every additional year of education conferring nearly a two‐point increase in ELC scores. FM and EL scores were positively associated with age at testing and gestational age, though the effect sizes were very small. Sex effects were more pronounced, with males scoring approximately one point lower on FM and VR, and nearly five points lower on the ELC at age 2. Gestation number significantly impacted 2‐year ELC and RL scores, with twins scoring 5.6 points and 1.24 points lower than singletons, respectively.
Cognitive scores were significantly correlated between ages 1 and 2. ELC scores at age 1 explained 7.3% of the variance in 2‐year ELC scores (r = .27, n = 335). Domain specific scores explained between 5.8 and 11.6% of the variance in future domain‐specific performance, with FM scores being the most correlated over time (r = .34, n = 336) and RL scores being the least correlated over time (r = .24, n = 336).
For comparison to WM‐cognition associations, Pearson's correlations were generated between main demographic variables of interest and cognitive composite scores. Maternal education accounted for 16% of the variance in offspring ELC scores at age 2 even after adjusting for gestational age, gestation number, and child sex (r = .40, p < .0001), but was not associated with 1‐year ELC scores. Gestational age at birth accounted for 12% of the variance in 2‐year ELC scores for the full sample (r = .34, p < .0001), which was highly similar when considering twins alone (r = .34, p < .0001) but accounted for only 4% of the variation in 2‐year ELC scores in singletons (r = .21, p = .007) who were generally born at later gestational ages. Gestational age at birth was not found to significantly correlate with 1‐year ELC scores. Similarly, gestational number (i.e., one for singletons, two for twins) accounted for just over 2% of the variance in 2‐year ELC scores after controlling for gestational age and sex (r = −.16, p < .005), and was not significantly correlated with 1‐year ELC scores.
3.2. DTI parameters: developmental patterns and influences of demographic factors
Developmental trajectories of AD, RD, and FA are presented in Figure 2. The greatest changes in parameters are observed in the first year of life, with slower rates of maturation in the second year of life. AD and RD, as expected, decrease with age (Figure 2a,b) while FA increases with age (Figure 2c). In terms of the correlations of parameters across development, there are generally modest significant correlations of neonatal AD, RD, and FA across nearly all tracts with future parameter values at age 1 (AD: average correlation across tracts = 0.29, SD = 0.07, all p < .05; RD: average correlation = 0.28, SD = 0.06, all p < .05; FA: average correlation = 0.34, SD = 0.09, all p < .05). Correlations between age 1 and age 2 are much higher (AD: average correlation across tracts = 0.69, SD = 0.10, all p < .05; RD: average correlation = 0.72, SD = 0.05; FA: average correlation = 0.77, SD = 0.06, all p < .05). Within‐subject correlations between DTI parameters at each age reveal that AD and RD are highly correlated in neonates (average correlation = 0.88, SD = 0.05, all p < .05) and remain correlated in 1‐year‐olds (average correlation = 0.59, SD = 0.13, all p < .05) and 2‐year‐olds (average correlation = 0.51, SD = 0.14, all p < .05). Correlations between RD and FA are also strong in neonates (average correlation = −0.82, SD = 0.10, all p < .05), 1‐year‐olds (average correlation = −0.76, SD = 0.06, all p < .05) and 2‐year‐olds (average correlation = −0.74, SD = 0.06, all p < .05). Correlations between AD and FA are relatively low in neonates (average correlation = −0.52, SD = 0.14, all p < .05), restricted to only a few tracts at age 1 where the direction of effect varies (min correlation = −0.17, max correlation = 0.54, SD = 0.30, all p < .05), and are only significant in a few tracts at age 2 (average correlation = 0.29, SD = 0.13, all p < .05).
Figure 2.
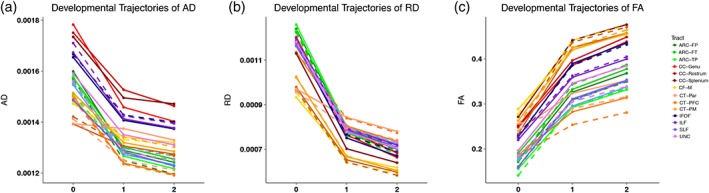
Developmental trajectories of white matter tracts. Developmental trajectories of AD (a), RD (b), and FA (c) for individual tracts across the first two years of life. Trajectories for left‐hemisphere tracts are shown as dotted lines [Color figure can be viewed at http://wileyonlinelibrary.com]
Neonatal AD and RD across tracts are very highly correlated (Figure 3a,b), while FA correlations between tracts are somewhat weaker overall, with the bilateral CGC and the splenium being less correlated with other tracts (Figure 3c). By age 1, correlations begin to weaken, and patterns of between‐tract correlations emerge for AD and FA, such that arcuate tracts, corticofugal, and some corticothalamic tracts, the IFOF and ILF, SLF and arcuate tracts, and SLF, UNC, and Genu are more strongly correlated with each other than with other tracts (Figure 3a,c). RD correlations remain relatively homogeneous across most tracts, though the left CT‐PFC is less correlated with other tracts (Figure 3b). We also found the inter‐subject variability in DTI metrics is significantly lower at ages 1 (AD: t = 11.35, p < .0001; RD: t = 15.64, p < .0001; FA: t = 2.76, p = .008) and 2 (AD: t = 11.69, p < .0001; RD: t = 15.62, p < .0001; FA: t = 1.84, p = .04) than in the neonatal period.
Figure 3.
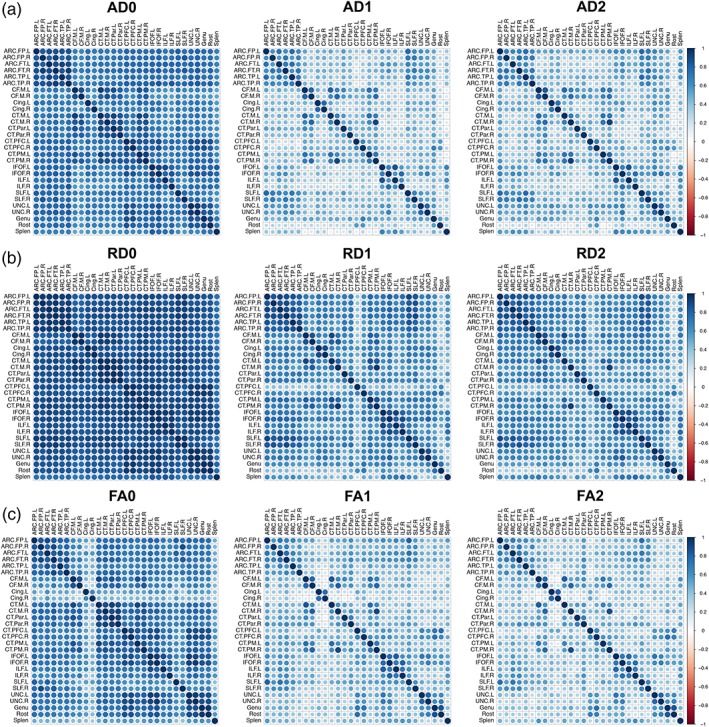
Correlations between tracts over time by DTI parameters. Correlations between tracts for AD (a), RD (b), FA (c) at the neonatal time‐point, age 1, and age 2 are shown. Colors represent correlation values from −1 to 1, where negative associations are shown in red and positive associations are shown in blue [Color figure can be viewed at http://wileyonlinelibrary.com]
With regards to associations with demographic factors, we found that neonatal FA, AD, and RD are strongly correlated with gestational age at birth, such that longer gestation reflects greater maturation (i.e., higher FA, lower AD, and RD). This was true for AD in all tracts (average correlation across tracts = −0.36, SD = 0.05, all p < .0001), RD in all tracts (average correlation = −0.39, SD = 0.05, all p < .0001), and FA in all tracts except the left CGC (average correlation = 0.29, SD = 0.08, all p < .03). These effects are largely not present at ages 1 and 2. Sex differences were observed in a few tracts in neonates and 1‐year‐old; females exhibited lower AD in the bilateral ILF and higher FA in the bilateral UNC following birth and lower AD in the bilateral ILF and splenium at age 1. Maternal education was not found to correlate with AD or RD at any age, though it was correlated with FA at age 1 in three tracts: the bilateral CF‐M (left: r = .18, p = .033; right: r = .20, p = .020) and the right CT‐Par (r = .20, p = .020).
3.3. Associations between WM and present and future cognition
3.3.1. ELC scores
There were no significant correlations between ELC scores at age 1 and neonatal FA, AD, or RD for any tract (Figure 4a–f). In contrast, there were widespread significant correlations across multiple tracts between ELC scores at age 2 and neonatal FA, AD, and RD (Figure 4a,c,e), though these associations do not survive adjustment for covariates including gestational age, gestation number, age at assessment, sex, maternal education, MSEL test date, and DTI protocol (Figure 4b,d,f). Correlations with AD and RD were negative, such that lower, or more mature values were associated with higher scores, while higher, more mature FA values following birth were associated with higher scores at age 2.
Figure 4.
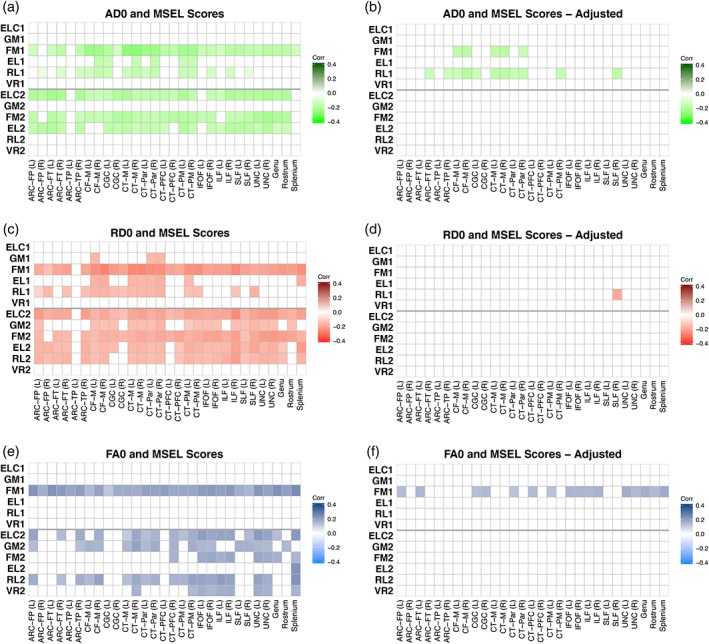
Correlations between DTI parameters after birth and cognitive scores at ages 1 and 2. Unadjusted correlations are shown between AD (a), RD (c), and FA (e) in neonates and general cognitive ability (ELC), gross motor (GM), fine motor (FM), expressive language (EL), receptive language (RL), and visual reception (VR) scores at ages 1 and 2. Adjusted correlations (corrected for gestational age at birth, sex, gestation number, maternal education, age at testing, MSEL test date, and scanner variables) between WM in the neonatal period and cognitive scores at ages 1 and 2 are shown for AD (b), RD (d), and FA (f). Colors represent Pearson's correlation values where brighter colors represent negative correlations and darker colors represent positive correlations; correlations that were nonsignificant after FDR correction are set to zero and shown as white [Color figure can be viewed at http://wileyonlinelibrary.com]
At age 1, significant cross‐sectional unadjusted correlations were found between ELC scores and FA in the left CT‐M and splenium (Figure 5e); associations which remained significant after adjustment for covariates. Interestingly, widespread cross‐sectional associations between ELC scores at age 1 and AD, RD, and FA spanning many tracts emerged after adjustment for covariates (Figure 5b,d,f). There were no significant cross‐sectional associations between WM microstructure at age 2 and ELC scores at age 2. A single negative association between 2‐year ELC scores and FA in the right CGC at age 1 was found (Figure 5e), though this finding was not significant after correcting for covariates.
Figure 5.
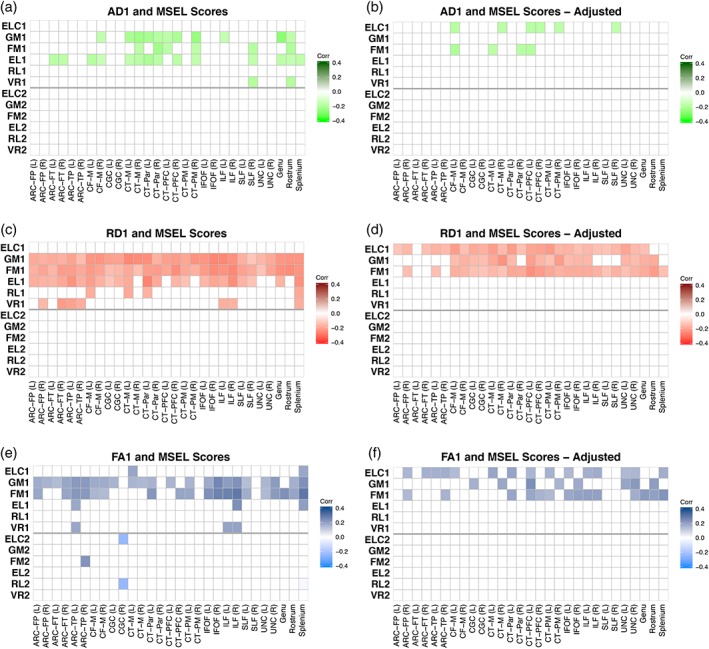
Correlations between DTI parameters at age 1 and cognitive scores at ages 1 and 2. Unadjusted correlations are shown between AD (a), RD (c), and FA (e) at age 1 and general cognitive ability (ELC), gross motor (GM), fine motor (FM), expressive language (EL), receptive language (RL), and visual reception (VR) scores at ages 1 and 2. Adjusted correlations (corrected for gestational age at birth, sex, gestation number, maternal education, age at testing, MSEL test date, and scanner variables) between WM at age 1 and cognitive scores at ages 1 and 2 are shown for AD (b), RD (d), and FA (f). Colors represent Pearson's correlation values where brighter colors represent negative correlations and darker colors represent positive correlations; correlations that were nonsignificant after FDR correction are set to zero and shown as white [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3.2. One‐year domain‐specific scores
There were widespread significant associations between FM scores at age 1 and neonatal FA, AD, and RD across most tracts (Figure 4a,c,e), though only a subset of correlations between FM scores at age 1 and tract‐based AD and FA remain significant after controlling for covariates (Figure 4b,f). In addition, there were several significant associations between EL and RL scores at age 1 and neonatal AD and RD, especially in cortico‐thalamic and arcuate tracts, and the IFOF, ILF, SLF, and CGC; only associations between RL scores at age 1 and neonatal AD remain after covariate adjustment (Figure 4b), along with a significant correlation between neonatal RD in the right SLF and RL scores at age 1 (Figure 4d).
There were widespread cross‐sectional associations between GM and FM scores at age 1 and FA and RD at age 1 (Figure 5c,e); AD was less frequently associated with motor scores (Figure 5a). Many of these associations survive adjustment for covariates (Figure 5b,d,e). Additionally, EL scores at age 1 were associated with widespread AD, and especially RD, at age 1 (Figure 5a,c), though these results did not remain after adjustment for covariates (Figure 5b,d). As with the ELC scores, lower AD and RD and higher FA values were associated with higher 1‐year domain‐specific scores.
3.3.3. Two‐year domain‐specific scores
There were widespread associations between GM, FM, EL, and RL scores at age 2 and neonatal RD across many tracts (Figure 4c). Widespread correlations are also found between FM and EL scores at age 1 and neonatal AD (Figure 4a), and between GM and RL, and to a lesser extent VR and FM scores, at age 2 and neonatal FA (Figure 4e). However, none of these associations between 2‐year domain‐specific scores and neonatal WM properties are significant after adjustment for covariates (Figure 4b,d,f). Significant associations between FA at age 1 in the right CGC and RL scores at age 2 and FA at age 1 in the right ARC‐TP and FM scores at age 2 (Figure 5e), though these associations were no longer significant after adjusting for covariates (Figure 5f). Interestingly, after adjusting for covariates, RL scores at age 2 were positively associated with AD at age 2 in the left ARC‐FP and left SLF (Figure 6). In general, lower AD and RD and higher FA values were associated with higher 2‐year domain‐specific scores, though there were a few exceptions to this pattern.
Figure 6.
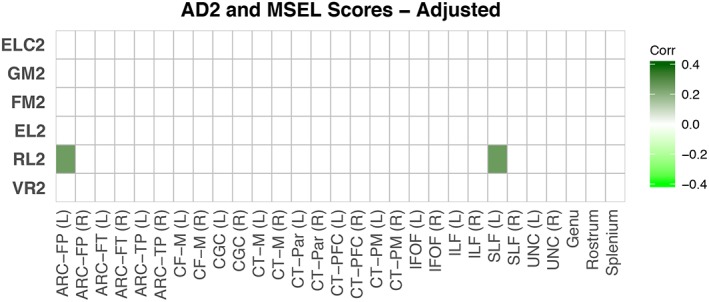
Correlations between AD at age 2 and cognitive scores at age 2. Adjusted correlations (corrected for gestational age at birth, sex, gestation number, maternal education, age at testing, MSEL test date, and scanner variables) between AD at age 2 and cognitive scores age 2 are shown. Colors represent Pearson's correlation values where brighter colors represent negative correlations and darker colors represent positive correlations; correlations that were nonsignificant after FDR correction are set to zero and shown as white [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Mediation analysis
Analyses testing for the mediating effect of neonatal WM on the association between gestational age and ELC scores at age 2 returned no significant results for any tract, and thus including neonatal measures of FA, AD, or RD had no impact on the association between gestational age and 2‐year ELC scores. This suggests that gestational age at birth influences 2‐year ELC scores through some other mechanism than by shaping neonatal WM. Mediation analyses were conducted on the entire sample, a subset of subjects excluding one twin from each pair, and the singletons only—results were highly similar between models. Results from the full sample can be seen in the Supporting Information.
3.5. Longitudinal changes in WM and 2‐year cognitive scores
Mixed effects models testing for the relationships between WM properties shortly after birth (FA0, AD0, and RD0), the change in WM properties from neonate to age 1 (dFA1,0, dAD1,0, and dRD1,0), and the change from age 1 to age 2 (dFA2,1, dAD2,1, and dRD2,1), while controlling for covariates, revealed that the developmental patterns of several tracts were related to 2‐year ability (Table 5). Higher ELC scores were associated with a slower decrease in AD from 1 to 2 years in the right SLF. Higher GM scores associated with a slower increase in FA in the first year of life in the left UNC. Higher RL scores at age 2 were related to slower decreases in RD from 1 to 2 years in several tracts including the bilateral ARC‐FT, right ARC‐TP, bilateral CF‐M, left CGC, bilateral CT‐M, right CT‐Par, right CT‐PM, left IFOF, left ILF, and left SLF. Higher RL scores were also associated with a faster decrease from 1 to 2 years in RD in the left CT‐PFC.
Table 5.
Developmental changes in DTI parameters relate to cognitive scores at age 2
| Tract | MSEL Score | Effect | Estimatea | Interpretationb | Std. Errorc | DFd | FDR p e | N Subsf | N Obsg |
|---|---|---|---|---|---|---|---|---|---|
| SLF (R) | ELC2 | dAD2,1 | 2.02E + 05 | Slower decrease, higher score | 5.99E + 04 | 38.39 | .050 | 53 | 56 |
| UNC (L) | GM2 | dFA1,0 | −6.83E + 01 | Slower increase, higher score | 5.18E + 00 | 4.78 | .002 | 59 | 65 |
| ARC‐FT (L) | RL2 | dRD2,1 | 4.34E + 04 | Slower decrease, higher score | 1.48E + 04 | 16.89 | .026 | 59 | 65 |
| ARC‐FT (R) | RL2 | dRD2,1 | 5.75E + 04 | Slower decrease, higher score | 2.19E + 04 | 26.13 | .035 | 59 | 64 |
| ARC‐TP (R) | RL2 | dRD2,1 | 4.92E + 04 | Slower decrease, higher score | 1.75E + 04 | 33.99 | .026 | 59 | 66 |
| CF‐M (L) | RL2 | dRD2,1 | 4.15E + 04 | Slower decrease, higher score | 1.10E + 04 | 7.85 | .024 | 59 | 66 |
| CF‐M (R) | RL2 | dRD2,1 | 4.57E + 04 | Slower decrease, higher score | 1.04E + 04 | 7.21 | .018 | 59 | 65 |
| CGC (L) | RL2 | dRD2,1 | 3.87E + 04 | Slower decrease, higher score | 1.60E + 04 | 42.83 | .042 | 57 | 63 |
| CT‐M (L) | RL2 | dRD2,1 | 4.11E + 04 | Slower decrease, higher score | 9.38E + 03 | 7.20 | .018 | 60 | 66 |
| CT‐M (R) | RL2 | dRD2,1 | 5.76E + 04 | Slower decrease, higher score | 1.41E + 04 | 5.51 | .026 | 60 | 66 |
| CT‐Par (R) | RL2 | dRD2,1 | 2.95E + 04 | Slower decrease, higher score | 1.14E + 04 | 16.78 | .042 | 60 | 66 |
| CT‐PM (R) | RL2 | dRD2,1 | 5.05E + 04 | Slower decrease, higher score | 1.10E + 04 | 9.23 | .017 | 60 | 66 |
| IFOF (L) | RL2 | dRD2,1 | 3.88E + 04 | Slower decrease, higher score | 9.56E + 03 | 9.84 | .018 | 60 | 66 |
| ILF (L) | RL2 | dRD2,1 | 2.79E + 04 | Slower decrease, higher score | 7.41E + 03 | 23.26 | .017 | 56 | 60 |
| SLF (L) | RL2 | dRD2,1 | 3.40E + 04 | Slower decrease, higher score | 9.26E + 03 | 9.12 | .024 | 57 | 62 |
| CT‐PFC (L) | RL2 | dRD2,1 | −2.19E + 04 | Faster decrease, higher score | 6.23E + 03 | 7.02 | .026 | 60 | 65 |
Model estimate.
Interpretation: higher MSEL scores at age 2 are predicted by slower decreases over time in AD or RD (positive estimates), slower increases in FA (negative estimates), faster decreases in AD or RD, or greater FA at birth (positive estimate).
Standard error.
Degrees of freedom.
FDR‐corrected p‐value.
Number of unique subjects in the analysis.
Number of total subjects in the analysis, treating one twin from each pair as repeated measures.
3.6. Sensitivity analyses
Sensitivity analyses splitting the sample into full‐term and preterm infants revealed similar trends between the two groups, though statistical significance was rarely met in either group, likely due to the reduction in sample size and reduced power to detect effects. In terms of observed trends in the data, preterm infants tended to have somewhat stronger associations between WM integrity at birth and VR scores at age 2 that were not picked up in the full‐term sample, generally stronger associations between WM integrity at age 1 and concurrent cognition than noted in singletons, and there was somewhat more variability in the direction of effect for the results in the cross‐sectional and longitudinal models for preterm infants, though it should be noted that nearly all of these results were nonsignificant (for details see Supporting Information).
Analyses splitting the sample into twins and singletons also revealed similar results. Most correlations were of a similar magnitude and direction, though they did not always reach statistical significance, likely due to a reduction in sample sizes. Additionally, correlations in the twin group were somewhat higher than in the singleton group (for details see Supporting Information).
Linear mixed models treating each twin from a twin‐pair as repeated measures were conducted to account for the relatedness of twins. The results from these analyses are highly similar to those from partial correlations, though fewer FA results are found to be significant, possibly due to the reduction in the number of unique subjects, and the ELC at age 2 was found to be positively related to AD in the left SLF (see Supporting Information for a summary of results).
Pearson's correlations between WM and cognition were corrected for age and sex only and compared to unadjusted correlations. Moderate to full overlap and similar trends between the models were found when probing relationships between WM at birth and cognitive scores at age 1, WM at age 1 and concurrent cognitive scores, WM at age 1 and scores at age 2, and WM at age 2 and concurrent scores (see Supporting Information). This suggests that while including age and sex as covariates do account for some of the association between WM and cognition, WM itself accounts for unique variation in cognition beyond age and sex in most cross‐sectional and longitudinal comparisons. We did find that associations between neonatal WM and 2‐year cognitive scores, except gross motor scores, were no longer significant after controlling for gestational age and sex. This is no surprise as gestational age accounts for significant variation in both neonatal WM and 2‐year cognitive scores, though we found that neonatal WM does not mediate the relationship between gestational age and 2‐year cognitive scores, implicating a separate mechanism by which gestational age impacts toddler cognitive ability. Again, this suggests that the unadjusted associations between WM and cognition are not simply driven by age‐related factors.
4. DISCUSSION
In the present study, we found widespread associations between tract‐average measures of FA, AD, and RD in the first year of life and cognitive outcomes at ages 1 and 2, suggesting that WM microstructure in infancy is associated with emergent cognition. Results indicated that generally higher FA and lower AD and RD, as a reflection of more mature WM, confer cognitive benefits in early life. The majority of our findings were with RD, suggesting that it may be a particularly important marker of individual differences in WM maturation during this developmental window. Additionally, we found that generally slower rates of change in RD in the second year of life across several fiber tracts, possibly reflecting more protracted myelination, related to better receptive language scores at age 2. After adjusting for variables associated with WM and cognition, neonatal DTI parameters did not appear to be strong biomarkers of cognition in infancy, though correlations between FA, AD, and RD at age 1 and concurrent ELC and gross and fine motor scores remained significant, suggesting a fairly strong association between WM integrity at age 1 and concurrent cognition. Taken together, our findings suggest that early postnatal WM integrity across many tracts in the brain, as a reflection of global coordinated WM development, are important for infant cognition and highlight that the role of WM development in cognitive development may be age‐dependent and influenced by child and demographic factors.
4.1. WM spanning the brain is associated with cognition across domains in infancy
Generally, we found widespread relationships between WM tracts and cognitive scores, suggesting that global brain WM integrity is related to cognition at early ages, as opposed to tract‐specific associations. This is particularly true for associations between neonatal WM and cognition in infancy, as fine motor, expressive language, and receptive language scores are all correlated with similar tracts, which include arcuate language tracts, sensory relay projection tracts, and higher‐order association tracts. The same is true for the correlations between all motor and language scores at age 1 and concurrent WM integrity. Such widespread associations are not surprising as we demonstrated that microstructure is highly correlated across tracts in early postnatal life, and previous work suggests that principal components explaining variance in WM properties across tracts in infancy are associated with emergent cognition (Lee et al., 2017). The global nature of our findings may reflect the highly plastic nature of the infant brain, in that tracts have yet to develop functional specificity. Alternatively, widespread associations found between WM integrity and infant cognition may also be attributable to the difficulty of assessing individual cognitive constructs in infancy which often depends on language skills for instruction and response, as well as fine motor skills. Tapping more specific cognitive domains may allow for the detection of domain‐specific findings in early infancy as have been reported by Short et al. (2013) in their study of tracts associated with working memory in 1‐year‐old.
The majority of the significant adjusted correlations were found between WM at age 1 and concurrent cognition, while the majority of significant unadjusted correlations were found between neonatal WM measures and subsequent cognition. This suggests that WM microstructure in the first year of life carries particularly important information for proximal infant cognitive development. The lack of many significant brain‐cognition relationships between WM at ages 1 and 2 and general cognition at age 2 is puzzling given that cognitive ability at age 2 is more closely associated with school‐age intelligence (Girault et al., 2018), though it could be attributed to reduced power to detect effects from smaller sample sizes. Alternatively, given that the standard deviation in FA, AD, and RD among individuals is significantly lower at ages 1 and 2 than at shortly after birth, is possible that individual differences between WM integrity at these ages lack the ability, on their own, to account for significant variation in cognitive scores at age 2. Additionally, we found that demographic factors were more strongly correlated with 2‐year scores than 1‐year scores, which when added to the model rendered correlations between neonatal WM integrity and 2‐year scores nonsignificant—this could indicate that the 1‐year measures capture information about the cognitive state of infants as opposed to the trait‐like features of 2‐year scores which are associated with later measures and influenced by demographic variables. Finally, it is also possible that WM integrity at ages 1 and 2 represent transient individual differences without long‐term consequences.
4.2. More mature fiber bundles in the first year relate to better cognitive performance
As expected, we found that better cognitive scores are associated with greater levels of maturation in fiber bundles (lower RD and AD, higher FA) in the first year of life. Our findings, and those from previous work (Geng et al., 2012), demonstrate that AD, RD, and FA develop rapidly in early life as a reflection of changes in the underlying WM microstructure. By 30 weeks gestation, major hubs in the WM network are in place (Ball et al., 2014) and by the time of birth, major fiber bundles are present, organized, and “adult‐like” in their structure, enough so to be reliably reproduced in our study and others (Dubois et al., 2008; Dubois, Hertz‐Pannier, Dehaene‐Lambertz, Cointepas, & Le Bihan, 2006; Hermoye et al., 2006). While myelination is the dominant process driving WM development in the early postnatal period, other factors also contribute to changes in diffusion signal, including membrane proliferation, changes in axonal diameter, and increasingly dense fiber packaging (Dubois et al., 2014), making it difficult to identify the exact mechanism driving the observed associations between WM development and cognition.
Of all significant unadjusted and adjusted correlations, RD accounted for the largest proportion of findings, while AD and FA accounted for equal portions of the remaining findings. Dubois et al. (2014) posit that, while FA and AD are good markers of fiber organization and are sensitive to characterizing fiber compactness and structure, the interpretation of their changes across early development may be difficult to discern given that they may increase or decrease in response to various developmental events including premyelination (FA increases, AD increases), myelination (FA increases, AD could decrease or remain the same), and the myelination of crossing fibers (FA decreases as the secondary bundle myelinates, AD decreases). On the other hand, RD consistently decreases across the different stages of WM development, making it a relatively strong marker of overall maturation, particularly during infancy (Dubois et al., 2014), and thus our findings between WM and cognition in infancy may be driven by a variety of neurodevelopmental mechanisms which are captured by RD. It is important to note, however, that previous work identified that common factors of AD in neonates, 1‐year‐old, and 2‐year‐old were related to cognitive scores at ages 1 and 2 whereas common factors of FA and RD at only age 1 were related to cognition at the same age (Lee et al., 2017), suggesting there may be age‐specific associations between DTI parameters and cognition. Additionally, we found strong associations between AD and RD across development, and overlapping results between these parameters in our data, suggesting that they may both be important for cognition in this period.
4.3. Protracted development of WM in the second year relates to better cognitive performance
Generally slower decreases in RD from age 1 to 2, and to a lesser extent AD, were associated with better cognitive scores, namely receptive language scores. This suggests that a prolonged period of WM development and plasticity may be beneficial for building WM pathways that better support subsequent cognitive development, and in this case particularly language development, in young children. However, opposite trends were observed in some tracts, and longitudinal findings were not significant when splitting the sample into full‐term, preterm, and single‐born participants, suggesting the effects may be tract‐specific and follow developmental patterns that vary across the brain or may vary across populations.
In support of these longitudinal findings, higher AD in the left ARC‐FP and SLF were associated with receptive language scores at age 2. These two tracts run parallel to each other and have been implicated in language (Dick & Tremblay, 2012). This negative association between AD and infant cognition has been observed before (Lee et al., 2017) and may suggest that children with more protracted AD development in language tracts perform better on these assessments. Interestingly, this also provides evidence to support the hypothesis that tracts may develop functional specificity over time through interactive specialization (M. H. Johnson, 2000; Redcay, Haist, & Courchesne, 2008; Swanson et al., 2015).
4.4. The contribution of demographic factors to WM microstructure and cognition
Our findings revealed that maternal education level was the single strongest predictor of offspring cognitive abilities at age 2, explaining 16% of the variance in 2‐year ELC scores. In comparison, gestational age at birth explained 4% of the variance in ELC scores in singletons and 12% in twins, and WM properties explained between roughly 2–6% of the variation in scores at age 2. The notable impact of maternal education level, and by proxy socioeconomic status, on child development is no surprise as there is a rich literature describing the association between maternal education and child behavior (Bornstein, Hahn, Suwalsky, & Haynes, 2003) and executive functioning (Rochette & Bernier, 2014). Whether these advantages are inherited (Bishop et al., 2003; Deary, Johnson, & Houlihan, 2009) fostered through increased access to resources and the utilization of effective parenting practices (Bernier, Carlson, & Whipple, 2010; Lugo‐Gil & Tamis‐LeMonda, 2008), or some combination of these pathways, maternal education level remains an important predictor of child developmental outcomes and deserves attention in studies of children's brain development. While our findings did not demonstrate a link between maternal education level and offspring WM development as measured by DTI tractography, other work suggests that maternal education and other indices of socioeconomic status impact brain development, and in particular cortical development (Hanson et al., 2013; Jha et al., 2018; Noble et al., 2015).
Gestational age at birth was found to have an important contribution to both neonatal WM integrity and infant cognitive ability at age 2, though we found no evidence of a mediation, suggesting that gestational age influences cognitive abilities in toddlers through some mechanism other than WM development. Strong associations between gestational age and WM development (Dubois et al., 2014; Geng et al., 2012; Partridge et al., 2004), and gestational age and children's cognitive development (Bode, D'Eugenio, Mettelman, & Gross, 2014) have been shown previously. We found that the impact of gestational age at birth was generally limited to proximal neonatal brain development, though some studies suggest differences in WM properties between preterm and full‐term infants may persist into childhood (Constable et al., 2008; Yung et al., 2007).
4.5. WM integrity as a biomarker of cognition
After correcting for covariates associated with WM development and early cognition, neonatal WM no longer remains significantly associated with 2‐year outcomes, concurrent brain‐cognition relationships at age 1 either emerge or remain largely similar, and several functionally specific relationships emerge at age 2 between tract WM and receptive language scores, suggesting that the usefulness of WM as a biomarker varies over time. The age‐specific nature of the relationships between WM and cognition in the first two years of life were also noted in a previous study (Lee et al., 2017). This varying nature in brain‐cognition relationships across development may be due to potential differences in the impact of covariates on cognition over time, functional specialization of tracts over time, the differences in the ability of infant measures to capture cognitive continuity across ages, or simply limitations of our neuroimaging measures to capture subtle, but dynamic brain–cognition relationships in a single snapshot.
The effect sizes noted in our study are relatively weak, with the absolute value of adjusted correlations between WM tract properties and infant and toddler cognitive scores ranging from 0.13 to 0.25, explaining between 1.7 and 6.3% of the variance in infant and toddler ability. Importantly, however, these effect sizes are nearly identical to those from a previous publication using a factor analysis approach (Lee et al., 2017), and very similar to those reported in older adults between general factors explaining WM and processing speed (Penke et al., 2010). Effect sizes reported in studies linking WM to working memory in 1‐year‐old (Short et al., 2013) and adults (Takahashi et al., 2010) show slightly higher correlations ranging from roughly 0.3 to 0.5. Taken together, these findings suggest that WM microstructure has very modest associations with general ability and slightly higher associations with specific domains such as working memory, but interestingly, the magnitude of these associations appear to be relatively stable across development.
4.6. Limitations
Results should be considered in the context of several limitations. Interpreting measures of WM microstructure derived from DTI is not trivial. While we have some knowledge as to how FA, AD, and RD develop over time, and how they may relate to primary developmental processes such as fiber organization, premyelination, and myelination, we cannot be certain that other confounding factors such as crossing fibers, especially the myelination of crossing fibers, and partial volume effects are not influencing the diffusion of water molecules in the brain (Dubois et al., 2014; Vos, Jones, Viergever, & Leemans, 2011); such technical limitations may contribute to opposite effects noted between DTI parameters and cognition at certain ages. The quantitative tractography method utilized in this study was well suited to discern specific brain–cognition associations, however the majority of our results did neither show tract‐specific functionality, nor very many parameter‐specific trends, and therefore it may be more fruitful to characterize WM development in early life using other approaches which may provide better descriptions of global WM structure. The generalizability of our study may also be impacted by the relatively large sample of twin‐born subjects and premature infants in our data set, as brain–cognition relationships may be different in these groups compared to full‐term, single‐born children, though we found similar general patterns between these groups in our data set. Finally, there are inherent limitations with studying infants and toddlers with regards to both imaging techniques and cognitive assessments. Motion in the scanner is always a concern, and to mitigate these effects we performed rigorous automated and manual quality control on both the scans and individual tracts from each subject and excluded subjects with data that were not of usable quality. Cognitive assessments are often difficult to collect in young children due to limited language and variable temperament, however our data were collected by experienced testers and independently reviewed to ensure that scores reflect child ability.
5. CONCLUSIONS AND FUTURE DIRECTIONS
In this study, we extended findings from our previous report (Lee et al., 2017) to further suggest that global WM development in infancy, as opposed to tract‐specific development, is associated with emerging cognition across domains in infancy and toddlerhood. We also report that tract‐based measures of WM in infancy and toddlerhood accounted for relatively little variance in child cognitive scores when compared to other readily available factors, especially maternal education level which accounted for the largest amount of variation in child cognitive scores. Our findings suggesting that it is of critical importance to consider contextual factors in the study of brain‐cognition associations.
Future research employing methods aimed at assessing brain‐wide WM organization and development is needed to replicate these findings and better characterize how global WM organization relates to cognitive development in early life and how individual difference factors shape such associations. Fruitful approaches may include data‐reduction approaches meant to capture overall patterns of variation in brain maturational status similar to that employed by Lee et al. (2017), as well as methods from the burgeoning field of connectomics research. Considering WM in the infant brain as a network allows for probing the global connectivity structure of underlying fiber pathways and does not necessitate the interpretation of diffusion metrics or the tracking of specific fiber pathways, providing additional complementary insight into how properties of the developing brain relate to the emergence of individual differences in cognitive abilities.
Supporting information
Appendix S1: Supplementary Material
ACKNOWLEDGMENTS
The authors thank our participating families and staff of the UNC NeuroImage and Research and Analysis Laboratories and the UNC Early Brain Development Study. A special thanks to those who helped with image processing and analysis: Joe Blocher, Shaili Jha, Mark Foster, and Rachel Steiner. The authors also thank the study coordinators, Dianne Evans, Jenny Prater, and Wendy Neuheimer. The authors are extremely grateful to all research assistants who collected the cognitive data over the course of the study: Elizabeth Misiti, Kathryn Cochran, Hillary Langley, Sarah Palmer, Portia Henderson, Molly McGinnis, Emily Bostwick, Sadie Hasbrouck, Monica Ferenz Guy, Kassidy Jezierski, Haley Parrish Black, Margo Williams, Margaret Hamilton Fox, Mallory Turner, and Emma Brink.
Girault JB, Cornea E, Goldman BD, Knickmeyer RC, Styner M, Gilmore JH. White matter microstructural development and cognitive ability in the first 2 years of life. Hum Brain Mapp. 2019;40:1195–1210. 10.1002/hbm.24439
Funding information: National Institute of Child Health and Human Development, Grant/Award Number: HD053000T32‐HD007376; National Institute of Mental Health, Grant/Award Number: MH070890
REFERENCES
- Ball, G. , Aljabar, P. , Zebari, S. , Tusor, N. , Arichi, T. , Merchant, N. , … Counsell, S. J. (2014). Rich‐club organization of the newborn human brain. Proceedings of the National Academy of Sciences of the United States of America, 111(20), 7456–7461. 10.1073/pnas.1324118111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, 57(1), 289–300. [Google Scholar]
- Bernier, A. , Carlson, S. M. , & Whipple, N. (2010). From External Regulation to Self‐Regulation: Early Parenting Precursors of Young Children's Executive Functioning. Child Development, 81(1), 326–339. [DOI] [PubMed] [Google Scholar]
- Bishop, E. G. , Cherny, S. S. , Corley, R. , Plomin, R. , Defries, J. C. , & Hewitt, J. K. (2003). Development genetic analysis of general cognitive ability from 1 to 12 years in a sample of adoptees, biological siblings, and twins. Intelligence, 31(1), 31–49. [Google Scholar]
- Bode, M. M. , D'Eugenio, D. B. , Mettelman, B. B. , & Gross, S. J. (2014). Predictive validity of the Bayley, Third Edition at 2 years for intelligence quotient at 4 years in preterm infants. Journal of Developmental and Behavioral Pediatrics : JDBP, 35(9), 570–575. 10.1097/DBP.0000000000000110 [DOI] [PubMed] [Google Scholar]
- Bornstein, M. H. , Hahn, C. S. , Suwalsky, J. , & Haynes, O. M. (2003). Socioeconomic status, parenting, and child development: The Hollingshead Four‐Factor Index of Social Status and the Socioeconomic Index of Occupations In Bornstein M. H. & Bradley R. H. (Eds.), Socioeconomic Status, Parenting, and Child Development (pp. 29–82). Mahwah, NJ: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Brody, B. A. , Kinney, H. C. , Kloman, A. S. , & Gilles, F. H. (1987). Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. Journal of Neuropathology and Experimental Neurology, 46(3), 283–301. [DOI] [PubMed] [Google Scholar]
- Constable, R. T. , Ment, L. R. , Vohr, B. R. , Kesler, S. R. , Fulbright, R. K. , Lacadie, C. , … Reiss, A. R. (2008). Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: An investigation of group and gender effects. Pediatrics, 121(2), 306–316. 10.1542/peds.2007-0414 [DOI] [PubMed] [Google Scholar]
- Deary, I. J. , Johnson, W. , & Houlihan, L. M. (2009). Genetic foundations of human intelligence. Human Genetics, 126(1), 215–232. 10.1007/s00439-009-0655-4 [DOI] [PubMed] [Google Scholar]
- Deoni, S. C. L. , Dean, D. C., III , O'Muircheartaigh, J. , Dirks, H. , & Jerskey, B. A. (2012). Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. NeuroImage, 63(3), 1038–1053. 10.1016/j.neuroimage.2012.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni, S. C. L. , Mercure, E. , Blasi, A. , Gasston, D. , Thomson, A. , Johnson, M. , … Murphy, D. G. M. (2011). Mapping infant brain myelination with magnetic resonance imaging. Journal of Neuroscience, 31(2), 784–791. 10.1523/JNEUROSCI.2106-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni, S. C. L. , O'Muircheartaigh, J. , Elison, J. T. , Walker, L. , Doernberg, E. , Waskiewicz, N. , … Jumbe, N. L. (2014). White matter maturation profiles through early childhood predict general cognitive ability. Brain Structure and Function, 221(2), 1189–1203. 10.1007/s00429-014-0947-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick, A. S. , & Tremblay, P. (2012). Beyond the arcuate fasciculus: Consensus and controversy in the connectional anatomy of language. Brain, 135(12), 3529–3550. 10.1093/brain/aws222 [DOI] [PubMed] [Google Scholar]
- Dubois, J. , Dehaene‐Lambertz, G. , Kulikova, S. , & Poupon, C. (2014). The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience, 276, 48–71. [DOI] [PubMed] [Google Scholar]
- Dubois, J. , Dehaene‐Lambertz, G. , Perrin, M. , Mangin, J.‐F. , Cointepas, Y. , Duchesnay, E. , … Hertz‐Pannier, L. (2008). Asynchrony of the early maturation of white matter bundles in healthy infants: Quantitative landmarks revealed noninvasively by diffusion tensor imaging. Human Brain Mapping, 29(1), 14–27. 10.1002/hbm.20363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois, J. , Hertz‐Pannier, L. , Dehaene‐Lambertz, G. , Cointepas, Y. , & Le Bihan, D. (2006). Assessment of the early organization and maturation of infants' cerebral white matter fiber bundles: A feasibility study using quantitative diffusion tensor imaging and tractography. NeuroImage, 30(4), 1121–1132. 10.1016/j.neuroimage.2005.11.022 [DOI] [PubMed] [Google Scholar]
- Fields, R. D. (2015). A new mechanism of nervous system plasticity: Activity‐dependent myelination. Nature Reviews Neuroscience, 16(12), 756–767. 10.1038/nrn4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, W. , Lin, W. , Chen, Y. , Gerig, G. , Smith, J. K. , Jewells, V. , & Gilmore, J. H. (2009). Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. AJNR. American Journal of Neuroradiology, 30(2), 290–296. 10.3174/ajnr.A1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng, X. , Gouttard, S. , Sharma, A. , Gu, H. , Styner, M. , Lin, W. , … Gilmore, J. H. (2012). Quantitative tract‐based white matter development from birth to age 2 years. NeuroImage, 61(3), 542–557. 10.1016/j.neuroimage.2012.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore, J. H. , Schmitt, J. E. , Knickmeyer, R. C. , Smith, J. K. , Lin, W. , Styner, M. , … Neale, M. C. (2010). Genetic and environmental contributions to neonatal brain structure: A twin study. Human Brain Mapping, 31(8), 1174–1182. 10.1002/hbm.20926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault, J. B. , Langworthy, B. W. , Goldman, B. D. , Stephens, R. L. , Cornea, E. , Steven Reznick, J. , … Gilmore, J. H. (2018). The predictive value of developmental assessments at 1 and 2 for intelligence quotients at 6. Intelligence, 68, 58–65. 10.1016/j.intell.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett, C. B. , Fletcher, P. T. , Gilmore, J. H. , & Gerig, G. (2009). Group analysis of DTI fiber tract statistics with application to neurodevelopment. NeuroImage, 45(1 Suppl), S133–S142. 10.1016/j.neuroimage.2008.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery, R. W. (2005). Is postnatal neocortical maturation hierarchical? Trends in Neurosciences, 28(10), 512–517. 10.1016/j.tins.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Hanson, J. L. , Hair, N. , Shen, D. G. , Shi, F. , Gilmore, J. H. , Wolfe, B. L. , & Pollak, S. D. (2013). Family poverty affects the rate of human infant brain growth. PLoS One, 8(12), e80954. 10.1371/journal.pone.0080954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoye, L. , Saint‐Martin, C. , Cosnard, G. , Lee, S.‐K. , Kim, J. , Nassogne, M.‐C. , … Mori, S. (2006). Pediatric diffusion tensor imaging: Normal database and observation of the white matter maturation in early childhood. NeuroImage, 29(2), 493–504. 10.1016/j.neuroimage.2005.08.017 [DOI] [PubMed] [Google Scholar]
- Jha, S. C. , Xia, K. , Ahn, M. , Girault, J. B. , Li, G. , Wang, L. , … Knickmeyer, R. C. (2018). Environmental influences on infant cortical thickness and surface area. Cerebral Cortex, 22, 1539 10.1093/cercor/bhy020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M. H. (2000). Functional brain development in infants: Elements of an interactive specialization framework. Child Development, 71(1), 75–81. [DOI] [PubMed] [Google Scholar]
- Kinney, H. C. , Brody, B. A. , Kloman, A. S. , & Gilles, F. H. (1988). Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. Journal of Neuropathology and Experimental Neurology, 47(3), 217–234. [DOI] [PubMed] [Google Scholar]
- Knickmeyer, R. C. , Gouttard, S. , Kang, C. , Evans, D. , Wilber, K. , Smith, J. K. , … Gilmore, J. H. (2008). A structural MRI study of human brain development from birth to 2 years. The Journal of Neuroscience, 28(47), 12176–12182. 10.1523/JNEUROSCI.3479-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer, R. C. , Xia, K. , Lu, Z. , Ahn, M. , Jha, S. C. , Zou, F. , … Gilmore, J. H. (2016). Impact of demographic and obstetric factors on infant brain volumes: A population neuroscience study. Cerebral Cortex, 27(12), 5616–5625. 10.1093/cercor/bhw331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. J. , Steiner, R. J. , Luo, S. , Neale, M. C. , Styner, M. , Zhu, H. , & Gilmore, J. H. (2015). Quantitative tract‐based white matter heritability in twin neonates. NeuroImage, 111, 123–135. 10.1016/j.neuroimage.2015.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. J. , Steiner, R. J. , Yu, Y. , Short, S. J. , Neale, M. C. , Styner, M. A. , … Gilmore, J. H. (2017). Common and heritable components of white matter microstructure predict cognitive function at 1 and 2 y. Proceedings of the National Academy of Sciences of the United States of America, 114(1), 148–153. 10.1073/pnas.1604658114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Nie, J. , Wang, L. , Shi, F. , Lyall, A. E. , Lin, W. , … Shen, D. (2014). Mapping longitudinal hemispheric structural asymmetries of the human cerebral cortex from birth to 2 years of age. Cerebral Cortex (New York, N.Y. : 1991), 24(5), 1289–1300. 10.1093/cercor/bhs413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo‐Gil, J. , & Tamis‐LeMonda, C. S. (2008). Family resources and parenting quality: Links to childrens cognitive development across the first 3 years. Child Development, 79(4), 1065–1085. 10.1111/j.1467-8624.2008.01176.x [DOI] [PubMed] [Google Scholar]
- Lyall, A. E. , Shi, F. , Geng, X. , Woolson, S. , Li, G. , Wang, L. , … Gilmore, J. H. (2015). Dynamic development of regional cortical thickness and surface area in early childhood. Cerebral Cortex (New York, N.Y. : 1991), 25(8), 2204–2212. 10.1093/cercor/bhu027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott, D. J. , Noseworthy, M. , Bouffet, E. , Laughlin, S. , & Rockel, C. (2006). White matter growth as a mechanism of cognitive development in children. NeuroImage, 33(3), 936–946. 10.1016/j.neuroimage.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Mamiya, P. C. , Richards, T. L. , Coe, B. P. , Eichler, E. E. , & Kuhl, P. K. (2016). Brain white matter structure and COMT gene are linked to second‐language learning in adults. Proceedings of the National Academy of Sciences of the United States of America, 113(26), 7249–7254. 10.1073/pnas.1606602113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen, E. M. (1995). Mullen Scales of Early Learning: AGS Edition. Circle Pines, MN: American Guidance Service, Inc. [Google Scholar]
- Nagy, Z. , Westerberg, H. , & Klingberg, T. (2004). Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience, 16(7), 1227–1233. 10.1162/0898929041920441 [DOI] [PubMed] [Google Scholar]
- Naigles, L. R. , Johnson, R. , Mastergeorge, A. , Ozonoff, S. , Rogers, S. J. , Amaral, D. G. , & Nordahl, C. W. (2017). Neural correlates of language variability in preschool‐aged boys with autism spectrum disorder. Autism Research, 44, 2221–1119. 10.1002/aur.1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble, K. G. , Houston, S. M. , Brito, N. H. , Bartsch, H. , Kan, E. , Kuperman, J. M. , … Sowell, E. R. (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18(5), 773–778. 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguz, I. , Farzinfar, M. , Matsui, J. , Budin, F. , Liu, Z. , Gerig, G. , … Styner, M. (2014). DTIPrep: Quality control of diffusion‐weighted images. Frontiers in Neuroinformatics, 8, 4 10.3389/fninf.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Muircheartaigh, J. , Dean, D. C. , Dirks, H. , Waskiewicz, N. , Lehman, K. , Jerskey, B. A. , & Deoni, S. C. L. (2013). Interactions between white matter asymmetry and language during neurodevelopment. The Journal of Neuroscience, 33(41), 16170–16177. 10.1523/JNEUROSCI.1463-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge, S. C. , Mukherjee, P. , Henry, R. G. , Miller, S. P. , Berman, J. I. , Jin, H. , … Vigneron, D. B. (2004). Diffusion tensor imaging: Serial quantitation of white matter tract maturity in premature newborns. NeuroImage, 22(3), 1302–1314. 10.1016/j.neuroimage.2004.02.038 [DOI] [PubMed] [Google Scholar]
- Penke, L. , Muñoz Maniega, S. , Murray, C. , Gow, A. J. , Hernández, M. C. V. , Clayden, J. D. , … Deary, I. J. (2010). A general factor of brain white matter integrity predicts information processing speed in healthy older people. The Journal of Neuroscience, 30(22), 7569–7574. 10.1523/JNEUROSCI.1553-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay, E. , Haist, F. , & Courchesne, E. (2008). Functional neuroimaging of speech perception during a pivotal period in language acquisition. Developmental Science, 11(2), 237–252. 10.1111/j.1467-7687.2008.00674.x [DOI] [PubMed] [Google Scholar]
- Rochette, E. , & Bernier, A. (2014). Parenting, family socioeconomic status, and child executive functioning: A longitudinal study. Merrill‐Palmer Quarterly, 60(4), 431 10.13110/merrpalmquar1982.60.4.0431 [DOI] [Google Scholar]
- Short, S. J. , Elison, J. T. , Goldman, B. D. , Styner, M. , Gu, H. , Connelly, M. , … Gilmore, J. H. (2013). Associations between white matter microstructure and infants' working memory. NeuroImage, 64, 156–166. 10.1016/j.neuroimage.2012.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel, M. E. (1982). Asymptotic confidence intervals for indirect effects in structural equation models. Sociological Methodolgy, 13, 290 10.2307/270723 [DOI] [Google Scholar]
- Swanson, M. R. , Wolff, J. J. , Elison, J. T. , Gu, H. , Hazlett, H. C. , Botteron, K. , … for the IBIS Network . (2015). Splenium development and early spoken language in human infants. Developmental Science, 20, e12360 10.1111/desc.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, M. , Iwamoto, K. , Fukatsu, H. , Naganawa, S. , Iidaka, T. , & Ozaki, N. (2010). White matter microstructure of the cingulum and cerebellar peduncle is related to sustained attention and working memory: A diffusion tensor imaging study. Neuroscience Letters, 477(2), 72–76. 10.1016/j.neulet.2010.04.031 [DOI] [PubMed] [Google Scholar]
- Takeuchi, H. , Sekiguchi, A. , Taki, Y. , Yokoyama, S. , Yomogida, Y. , Komuro, N. , … Kawashima, R. (2010). Training of working memory impacts structural connectivity. The Journal of Neuroscience, 30(9), 3297–3303. 10.1523/JNEUROSCI.4611-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde, A. R. , Budin, F. , Berger, J.‐B. , Gupta, A. , Farzinfar, M. , Kaiser, A. , … Styner, M. (2014). UNC‐Utah NA‐MIC framework for DTI fiber tract analysis. Frontiers in Neuroinformatics, 7, 51 10.3389/fninf.2013.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos, S. B. , Jones, D. K. , Viergever, M. A. , & Leemans, A. (2011). Partial volume effect as a hidden covariate in DTI analyses. NeuroImage, 55(4), 1566–1576. 10.1016/j.neuroimage.2011.01.048 [DOI] [PubMed] [Google Scholar]
- Wake, H. , Ortiz, F. C. , Woo, D. H. , Lee, P. R. , Angulo, M. C. , & Fields, R. D. (2015). Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nature Communications, 6, 7844 10.1038/ncomms8844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd, K. B. , Tamnes, C. K. , & Fjell, A. M. (2014). Brain structural maturation and the foundations of cognitive behavioral development. Current Opinion in Neurology, 27(2), 176–184. 10.1097/WCO.0000000000000074 [DOI] [PubMed] [Google Scholar]
- Yung, A. , Poon, G. , Qiu, D.‐Q. , Chu, J. , Lam, B. , Leung, C. , … Khong, P. L. (2007). White matter volume and anisotropy in preterm children: A pilot study of neurocognitive correlates. Pediatric Research, 61(6), 732–736. 10.1203/pdr.0b013e31805365db [DOI] [PubMed] [Google Scholar]
- Zatorre, R. J. , Fields, R. D. , & Johansen‐Berg, H. (2012). Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nature Neuroscience, 15(4), 528–536. 10.1038/nn.3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Material


