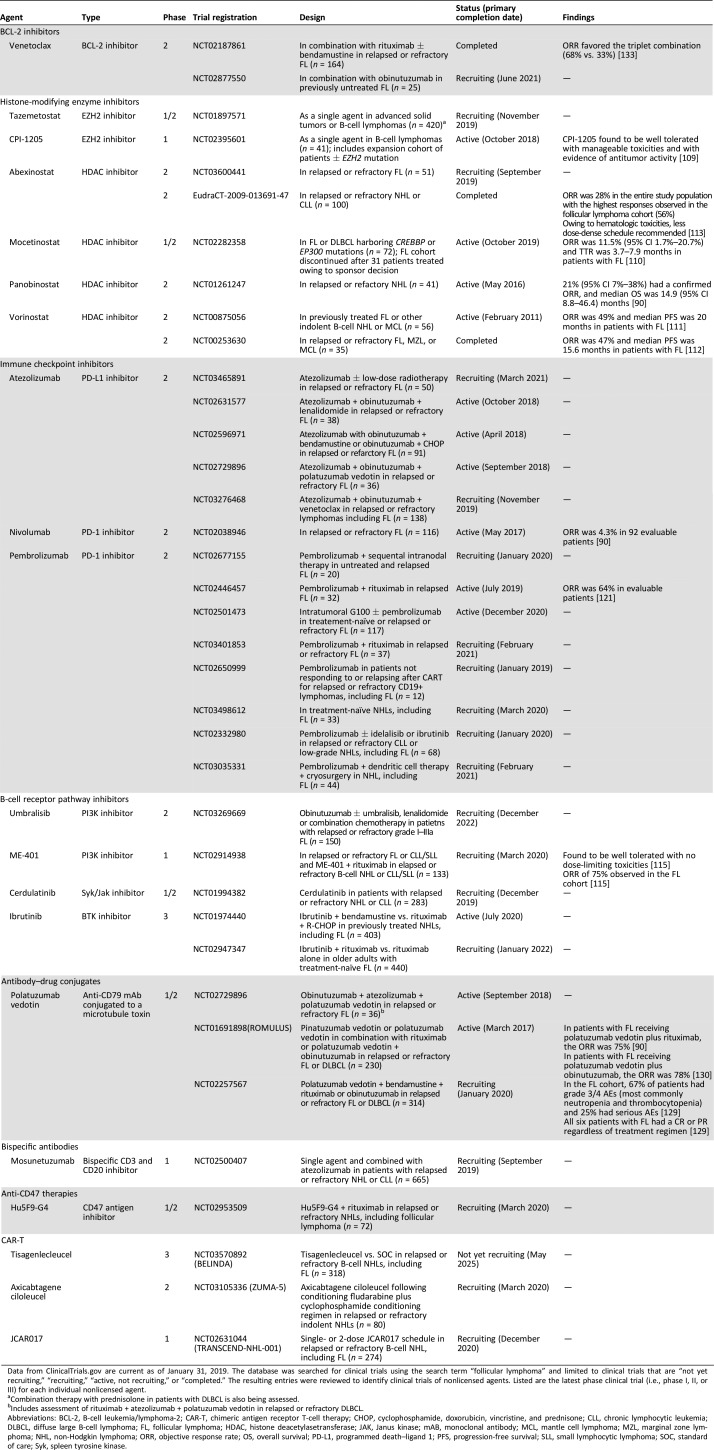
Table 2. Select clinical trials of investigational therapies in follicular lymphoma.
Data from ClinicalTrials.gov are current as of January 31, 2019. The database was searched for clinical trials using the search term “follicular lymphoma” and limited to clinical trials that are “not yet recruiting,” “recruiting,” “active, not recruiting,” or “completed.” The resulting entries were reviewed to identify clinical trials of nonlicensed agents. Listed are the latest phase clinical trial (i.e., phase I, II, or III) for each individual nonlicensed agent.
Combination therapy with prednisolone in patients with DLBCL is also being assessed.
Includes assessment of rituximab + atezolizumab + polatuzumab vedotin in relapsed or refractory DLBCL.
Abbreviations: BCL‐2, B‐cell leukemia/lymphoma‐2; CAR‐T, chimeric antigen receptor T‐cell therapy; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma; HDAC, histone deacetylasetransferase; JAK, Janus kinase; mAB, monoclonal antibody; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NHL, non‐Hodgkin lymphoma; ORR, objective response rate; OS, overall survival; PD‐L1, programmed death–ligand 1; PFS, progression‐free survival; SLL, small lymphocytic lymphoma; SOC, standard of care; Syk, spleen tyrosine kinase.