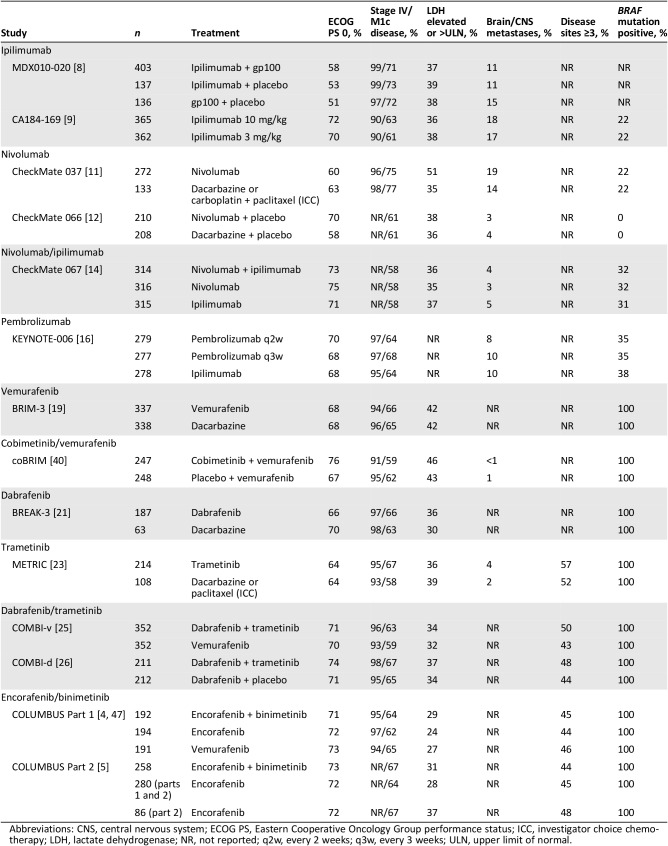
Table 2. Baseline characteristics for phase III trials.
Abbreviations: CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; ICC, investigator choice chemotherapy; LDH, lactate dehydrogenase; NR, not reported; q2w, every 2 weeks; q3w, every 3 weeks; ULN, upper limit of normal.