Abstract
Increased adiposity is associated with reduced skeletal muscle function in older adults, but the mechanisms underlying this relationship remain unclear. To explore whether skeletal muscle properties track with adiposity, whole-muscle, cellular, and molecular function were examined in relation to adiposity measured at various anatomical levels in healthy older (60–80 years) men and women. Although women had greater absolute and relative body and thigh fat than men, quadriceps muscle attenuation, an index of intramuscular lipid content, was similar between sexes. At the whole-muscle level, greater quadriceps attenuation was associated with reduced knee extensor function in women, but not men. In women, decreased myosin heavy chain I and IIA fiber-specific force was associated with higher intramuscular lipid content, which may be explained, in part, by the reduced myofilament lattice stiffness found in myosin heavy chain IIA fibers. Longer myosin attachment times in myosin heavy chain I fibers from men and women were associated with greater amounts of adipose tissue, suggesting that fat deposits lead to slower myosin–actin cross-bridge kinetics. Our results indicate greater quantities of adipose tissue alter myofilament properties and cross-bridge kinetics, which may partially explain the adiposity-induced decrements in single-fiber and whole-muscle function of older adults, especially women.
Keywords: Adiposity, Human, Muscle fiber, Myosin heavy chain
Altered body composition is a hallmark of human aging, characterized by a reduction in skeletal muscle mass (sarcopenia) and an increase in adiposity (1, 2). Age-related increases in adiposity are not confined to classical adipose tissue depots as lipids accumulate within various organs and tissues, including skeletal muscle (3). Accompanying these changes in body composition is a reduction in physical functional capacity (4, 5). Although reductions in muscle mass are thought to drive age-related functional deterioration, increasing adiposity may also contribute, as physical function is reduced among overweight and obese older adults relative to normal-weight counterparts (4, 5). Currently, nearly 40% of adults at least 60 years of age are classified as obese (6), a number that is likely to rise in coming decades. Thus, understanding how excess adiposity contributes to poor physical performance and disability in older adults has important public health implications.
A critical determinant of physical performance in older adults is skeletal muscle function (7), and a growing body of evidence indicates that muscle force and power is lower in overweight (body mass index [BMI] = 25.0–29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) older adults relative to their normal-weight counterparts per unit muscle size (8–11). The loss of function per unit muscle size in older adults is associated with a lower thigh muscle density (12), a marker of lipid accumulation, suggesting that lipid stored in the muscle reduces contractile function. One recent study showed that reduced specific power and unloaded shortening velocity in myosin heavy chain (MHC) I and IIA fibers correlated with greater intramyocellular lipid content in older adults (10), indicating a link between adiposity and muscle cell function. Elucidating the mechanisms responsible for impaired muscle contractility at the molecular level, such as altered myofilament mechanical properties and myosin–actin cross-bridge kinetics, will be important to identifying potential mechanisms whereby fat may alter muscle performance and in developing targeted countermeasures for obesity-induced skeletal muscle weakness and disability.
Skeletal muscle function scales up through various anatomical levels, as interactions between the primary myofilament proteins actin and myosin dictate single-fiber contractile properties (force, velocity, and power), which, in turn, influence whole-muscle function (strength and power) (13). Therefore, it is necessary to study the effects of adiposity at the molecular (myofilament mechanical properties and myosin–actin cross-bridge kinetics), cellular (single fiber–specific force), and whole-muscle (isometric and isokinetic torque/power) levels to elucidate how lipid alters muscle contractility. Toward this goal, the aim of the present study was to examine whole-body, thigh, quadriceps, and cellular adipose tissue deposition and its relationship with whole-muscle, cellular, and molecular contractile function in older adults. Because we have observed differences between older men and women in muscle structure and function at multiple anatomical levels (14–16), and in light of well-characterized sex differences in adiposity and tissue fat deposition (17, 18), we included sex in our analytical model.
Methods
Participants
Forty-four older men (n = 20) and women (n = 24) were evaluated in this study. This population of older adults was obtained by combining three data sets from our laboratories: (a) 12 older adults (5 men, 7 women) from our investigation into the effects of aging (14, 16); (b) 15 older controls (8 men, 7 women) from our investigation into the effects of disuse (15, 19); and (c) pre-exercise data from the 17 older adults (7 men, 10 women) from our investigation into the effects of moderate-intensity resistance exercise (20). Volunteers were recruited contemporaneously for these studies from 2008 to 2014. Participant activity level was discussed during screening and was confirmed by accelerometry to be sedentary (14, 16, 20) to moderately physically active (15, 19), with the exception of two older men for whom accelerometry was not performed. No volunteer was engaged in a structured exercise program and all women were postmenopausal. The 17 pre-exercise participants had radiographic and symptomatic evidence of knee osteoarthritis, but were otherwise healthy (20), and no participants had signs or symptoms of cardiovascular disease, diabetes, pulmonary disease, or neuromuscular disease, or a history of malignancy within the past 10 years (excluding nonmelanoma skin cancer). All participants provided written informed consent prior to enrollment in these studies. The protocols were approved by the Committees on Human Research at the University of Vermont.
Whole-Body and Thigh Composition
Body mass was measured using a digital scale (ScaleTronix, Wheaton, IL) and total and regional body composition was assessed via dual-energy x-ray absorptiometry (GE Lunar, Madison, WI). Thigh and quadriceps composition, including cross-sectional areas of muscle and intermuscular adipose tissue, and quadriceps attenuation were determined by computed tomography, as described (16). Intermuscular adipose tissue was defined as adipose tissue located beneath the deep fascia of the thigh and between the quadriceps and hamstrings muscle groups, consistent with the approach used in previous research (3). Quadriceps attenuation was used as a proxy for intramuscular lipid content (21).
Knee Extensor Muscle Function
Knee extensor torque and power were measured under isometric and isokinetic conditions using a dynamometer (Humac Norm, CSMi, Stoughton, MA). Isometric torque was measured during a maximal voluntary contraction at 70° of knee flexion and the peak torque from two attempts was used for analysis. Isokinetic power was measured during four repeated maximal voluntary contractions at 180°/s and peak power output was calculated during the isovelocity phase of the contraction.
Muscle Biopsy and Processing
A percutaneous needle biopsy of the vastus lateralis muscle was performed under lidocaine anesthesia and in the fasted state. The muscle tissue was processed and stored at −20°C until isolation of single fibers for mechanical measurements, which occurred within 4 weeks of the biopsy, as previously described (22).
Single-Fiber Mechanical Measurements
On the day of the experiment, muscle bundles were removed from storage and prepared for experiments, as described (22), including performing top- and side-diameter measurements at three positions along the length of the fiber to calculate average cross-sectional area. An initial maximal Ca2+ activation was performed to verify fiber integrity (maintenance of normal sarcomere register) and was performed at either 15°C and 0.25 mM Pi or 25°C and 5 mM Pi depending upon the study. Our aging study (14, 16) included an initial set of specific force measurements at 15°C and 0.25 mM Pi before sinusoidal analysis experiments at 25°C and 5 mM Pi, whereas our later studies (15, 19, 20) performed specific force measurements at 25°C and 5 mM Pi before sinusoidal analysis experiments under the same conditions. After initial maximal Ca2+ activation, the fiber was returned to relaxing solution and sarcomere length reset to 2.65 μm if necessary. Subsequent maximal Ca2+ activations went through the same procedure.
Myofilament properties and myosin–actin cross-bridge mechanics and kinetics were derived using sinusoidal analysis, as previously described (22), and were performed at 25°C under maximal Ca2+-activated conditions (pCa 4.5). This analysis yields three characteristic processes, A, B, and C, which relate to various mechanical (A, B, C, and k) and kinetic (2πb and 2πc) properties of the cross-bridge cycle, as described in detail (22). Briefly, the A-process (characterized by parameters A and k) describes a linear relationship between the viscous and elastic moduli that has no kinetic or enzymatic dependence (23). Under Ca2+-activated conditions, where myosin–actin cross-bridges are formed, the A-process represents the underlying stiffness of the lattice structure (myofilament lattice stiffness) and the attached myosin heads in series (23). The parameter A indicates the magnitude of a viscoelastic modulus and k describes the degree to which measured viscoelastic mechanics represents purely elastic (k = 0) versus purely viscous (k = 1) mechanical responses. The B- and C-process magnitudes (B and C) are proportional to the number of myosin heads strongly bound to actin and the cross-bridge stiffness (24). The frequency portion of the B-process (2πb) has been interpreted as the apparent (observed) rate of myosin force production or, in other words, the rate of myosin transition between the weakly bound and strongly bound states (25). The reciprocal of the frequency portion of the C-process, or (2πc)−1, represents the average myosin attachment time to actin, ton (24).
Myosin Heavy Chain Isoform Distribution
Following sinusoidal analysis measurements, single fibers were placed in 30 μL loading buffer, heated for 2 minutes at 65°C and stored at −80°C until determination of MHC isoform composition by sodium dodecyl sulfate–polyacrylamide gel electrophoresis to identify fiber type, as described (22). In addition, myofibrillar proteins were extracted from tissue homogenates and MHC isoform distribution determined by gel electrophoresis, as described (15, 19).
Intramyocellular Lipid Content
As quadriceps attenuation is a proxy for lipid infiltration, we measured intramyocellular lipid content using oil-red-o (ORO) immunohistochemistry, as described previously (26). Baseline samples (at least 4 images per participant) from 12 adults (n = 6 for men and n = 6 for women) enrolled in our prior study (20) were examined for cross-sectional area of individual lipid droplets, number of lipid droplets per area, and relative area occupied by lipid droplets, as the samples from other subgroups of this cohort were processed in a manner that precluded ORO experiments from being performed or had insufficient tissue for this analysis.
Intramyocellular lipid (cross-sectional area of individual lipid droplets, number of lipid droplets per area, and relative area occupied by lipid droplets) was also measured on samples of intact skeletal muscle fiber bundles using electron microscopy (EM), as described previously (27). For these analyses, n = 42 (data were missing for one male and one female participant) and at least 6 images were analyzed for each participant. Intramyocellular lipid droplets were identified according to the following criteria: a gray/white interior, somewhat circular in shape, contains a gray outline, lack of multiple membranes or debris, and typically located adjacent to mitochondria. The ORO and EM measurements were performed to yield overall lipid content and not determined for individual fiber types (eg, MHC I).
Statistical Analysis
Data are expressed as means ± SE. Normality of data was determined via skewness and kurtosis statistics and visual inspection of histograms and boxplots. An independent samples t-test was used to examine the main effect of sex on clinical characteristics and contractile properties of MHC I and IIA single fibers. Sex-specific Pearson correlations were conducted between measures of whole-muscle (isometric torque and isokinetic power), cellular (single fiber–specific force) and molecular (A, k, B, C, 2πb, and ton) contractile function, and amount of adipose tissue (percent body fat, thigh, and quadriceps intermuscular adipose tissue, quadriceps attenuation, and indices of intramyocellular lipid). Partial correlations adjusted for percent body fat were conducted to determine if relationships between regional adiposity and muscle function were independent of overall body fat. All statistical analyses were conducted using SPSS for Windows version 23.0 (IBM, Armonk, NY) and significance was considered p ≤ .05.
Results
Clinical Characteristics
Older women were shorter, weighed less, and had reduced habitual physical activity levels and knee extensor function compared to men (Table 1). Older women also had greater absolute and relative whole-body and intermuscular fat than men (Table 1). Despite greater adiposity, women had lower BMI than men, highlighting BMI as a relatively poor index for distinguishing adiposity between sexes. For this reason, we have restricted correlation analysis to more direct estimates of adiposity. Although women had greater thigh intermuscular adipose tissue, there was no difference between sexes in the relative quadriceps intermuscular adipose tissue, quadriceps muscle attenuation, or intramyocellular lipid content (Table 1).
Table 1.
Clinical Characteristics, Whole-Muscle Function, and Measures of Adiposity in Older Men (n = 20) and Women (n = 24)
| Men | Women | p | |||
|---|---|---|---|---|---|
| Average | Range | Average | Range | ||
| Age (y) | 68.8 ± 1.0 | 61.6–76.0 | 68.8 ± 1.0 | 61.3–78.6 | .99 |
| Height (cm) | 174.5 ± 1.3 | 162.1–182.8 | 158.5 ± 1.3 | 144.1–171.8 | <.01 |
| Weight (kg) | 82.6 ± 2.5 | 61.9–104.2 | 61.8 ± 2.2 | 48.2–83.9 | <.01 |
| Physical activity (kcal/d)† | 464.8 ± 43.0 | 206.9–784.9 | 337.5 ± 28.8 | 135.1–692.1 | .02 |
| Isometric torque at 70° (Nm)‡ | 188.9 ± 12.1 | 101.2–274.9 | 109.0 ± 7.3 | 39.5–188.7 | <.01 |
| Isokinetic power at 180°/s (W)‡ | 282.0 ± 20.9 | 168.8–484.7 | 161.3 ± 10.3 | 61.5–314.5 | <.01 |
| Body mass index (kg/m2) | 27.1 ± 0.6 | 21.9–31.7 | 24.6 ± 0.9 | 18.6–34.9 | .04 |
| Percent body fat (%) | 28.2 ± 1.4 | 14.1–38.9 | 37.7 ± 1.7 | 21.9–52.3 | <.01 |
| Thigh IMAT (cm2) | 84.1 ± 4.2 | 49.4–118.9 | 137.6 ± 10.0 | 71.3–229.2 | <.01 |
| Relative thigh IMAT (%) | 36.3 ± 1.2 | 23.9–43.1 | 58.0 ± 1.9 | 41.2–72.4 | <.01 |
| Thigh muscle (cm2) | 145.3 ± 3.7 | 113.7–182.9 | 93.1 ± 2.6 | 68.7–120.4 | <.01 |
| Quadriceps IMAT (cm2) | 4.1 ± 0.4 | 1.2–9.2 | 2.6 ± 0.2 | 1.2–5.0 | <.01 |
| Relative quadriceps IMAT (%) | 6.2 ± 0.7 | 1.8–14.3 | 6.2 ± 0.6 | 2.5–14.5 | 1.00 |
| Quadriceps muscle (cm2) | 62.4 ± 2.2 | 47.1–79.9 | 40.3 ± 1.4 | 27.8–52.0 | <.01 |
| Quadriceps attenuation (HU) | 51.4 ± 0.7 | 42.5–55.0 | 50.3 ± 0.8 | 42.8–57.8 | .33 |
| # of lipid droplets/um2, EM | 0.027 ± 0.003 | 0.007–0.058 | 0.021 ± 0.003 | 0.007–0.054 | .16 |
| Area fraction (%), EM | 0.60 ± 0.08 | 0.06–1.17 | 0.46 ± 0.37 | 0.04–1.68 | .21 |
| Lipid droplet size (um2), EM | 0.21 ± 0.02 | 0.10–0.37 | 0.19 ± 0.01 | 0.04–0.33 | .42 |
| # of lipid droplets/um2, ORO | 0.037 ± 0.004 | 0.021–0.052 | 0.028 ± 0.004 | 0.016–0.043 | .12 |
| Area fraction (%), ORO | 4.30 ± 0.48 | 2.38–5.78 | 3.00 ± 0.46 | 1.85–5.06 | .08 |
| Lipid droplet size (um2), ORO | 1.17 ± 0.10 | 0.86–1.45 | 1.07 ± 0.04 | 0.95–1.19 | .39 |
Notes: Data are presented as mean ± SE; EM: n = 19 for men and n = 23 for women; ORO: n = 6 for men and n = 6 for women. EM = electron microscopy; HU = Hounsfield units; IMAT = intermuscular adipose tissue; ORO = oil-red-o; # = number.
† n = 18 for men.
‡ n = 19 for men.
Relationships Between Whole-Muscle Function and Adiposity
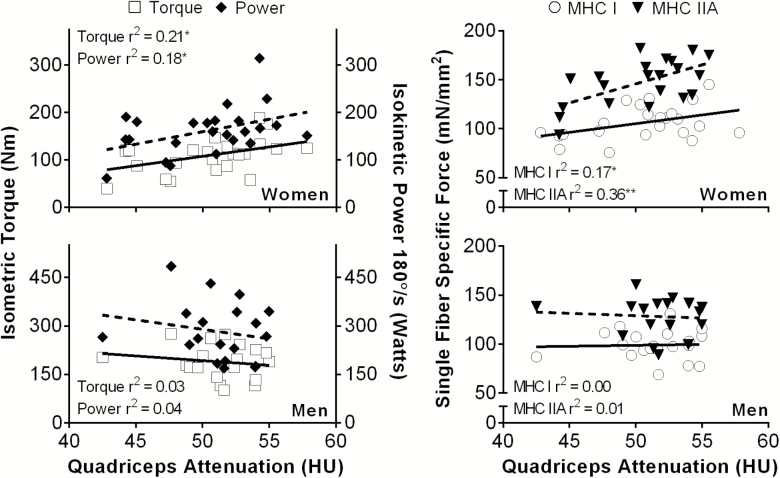
For comparison with previous studies (8–11), we first explored whether adiposity was associated with measures of whole-muscle function. When men and women were pooled, greater percent body fat was associated with lower isometric torque (r = −.47, p < .01) and isokinetic power (r = −.38, p = .01). Greater thigh intermuscular adipose tissue was also negatively correlated with whole-muscle function and had a similar strength of association (r range = −.40 to −.41, p < .01). Quadriceps attenuation was not associated with whole-muscle function until sex-specific analyses were conducted. In women, greater quadriceps attenuation was associated with higher peak isometric torque at 70° as well as isokinetic power at 180°/s; however, these relationships were not present in older men (Figure 1). Likewise, in women, percent body fat was negatively correlated with both isometric torque (r = −.55, p < .01) and isokinetic power (r = −.43, p = .04). In contrast, measures of adiposity were not associated with reduced whole-muscle function in older men.
Figure 1.
Relationships that (left) knee extensor function in older men (n = 19) and women (n = 24) and (right) specific force in myosin heavy chain (MHC) I and IIA single muscle fibers in older men (n = 18) and women (n = 21) had with intramuscular lipid content. *p ≤ .05; **p < .01.
Cellular and Molecular Contractile Properties
In MHC I fibers, specific force, myofilament lattice stiffness (A), cross-bridge number and stiffness (C), and myosin–actin cross-bridge kinetics (myosin attachment time [ton] and rate of myosin force production [2πb]) were similar between men and women (Table 2). In contrast, in MHC IIA fibers, specific force and myofilament lattice stiffness were higher in women, whereas myosin attachment time and rate of myosin force production remained unchanged compared to men (Table 2). As previous work in animals (28) and humans (29) has shown a slow-to-fast MHC isoform shift with obesity, we examined whether increasing levels of adiposity may affect changes in myofilament function, in part, by modulating myofilament protein expression. To accomplish this, we examined tissue homogenate MHC isoform composition. In addition to a similar MHC isoform distribution between sexes (Table 2), there were no relationships between any measure of adiposity and the percentage of MHC I (range of p values for men = .50–.85 and women = .08–.80) or MHC IIA (range of p values for men = .36–.84 and women = .31–.65) fibers in our cohort.
Table 2.
Contractile Properties of Myosin Heavy Chain (MHC) I and IIA Single Muscle Fibers From Older Men and Women
| MHC I | MHC IIA | |||||
|---|---|---|---|---|---|---|
| Men | Women | p | Men | Women | p | |
| Specific force (mN/mm2) | 99.1 ± 3.3 | 106.1 ± 3.6 | .17 | 128.3 ± 4.6 | 147.2 ± 5.2 | .01 |
| Myofilament lattice stiffness (N/mm2) | 945 ± 41 | 1,000 ± 40 | .34 | 2,092 ± 79 | 2,474 ± 124 | .02 |
| Cross-bridge number and stiffness (N/mm2) | 11.6 ± 1.1 | 12.6 ± 0.8 | .46 | 26.3 ± 1.4 | 29.3 ± 1.4 | .15 |
| Myosin attachment time (ms) | 33.5 ± 1.3 | 34.8 ± 1.4 | .50 | 16.5 ± 0.4 | 16.2 ± 0.3 | .60 |
| Rate of myosin force production (Hz) | 16.5 ± 0.6 | 16.9 ± 0.6 | .65 | 50.3 ± 1.1 | 49.7 ± 1.0 | .72 |
| MHC isoform composition (%) | 36.3 ± 1.0 | 37.3 ± 0.9 | .45 | 39.8 ± 0.7 | 39.3 ± 1.0 | .69 |
Note: MHC I: n = 20 for men and n = 23 for women. MHC IIA: n = 18 for men and n = 21 for women.
Relationships Between Cellular Function and Adiposity
The relationships between cellular contractile function and adiposity (quadriceps, thigh, and whole body) were examined with older men and women pooled, but no relationships were evident. However, correlation analyses performed within each sex produced numerous sex-specific relationships. In older women, lower maximally Ca2+-activated specific force in MHC I and IIA single fibers was associated with increased intramuscular fat, as reflected by reduced quadriceps attenuation (Figure 1). In addition, reduced specific force in MHC IIA fibers was associated with greater thigh intermuscular adipose tissue (r = −.55, p = .01) and percent body fat (r = −.67, p < .01), but not quadriceps intermuscular adipose tissue (p = .41). Specific force in MHC I fibers from older women did not correlate with thigh (p = .29) or quadriceps (p = .82) intermuscular adipose tissue or percent body fat (p = .37). In older men, specific force in MHC I and IIA fibers was not associated with quadriceps attenuation (p = .78–.87, Figure 1), thigh (p = .63–.67) or quadriceps (p = .63–.88) intermuscular adipose tissue, or percent body fat (p = .16–.44). Thus, in older women, force generation decreased in MHC I and IIA fibers with increased fat deposition in muscle and in MHC IIA fibers with amount of thigh and overall body fat, whereas, in older men, there were no relationships between force generation and any adiposity index.
Relationships Between Molecular Function and Adiposity
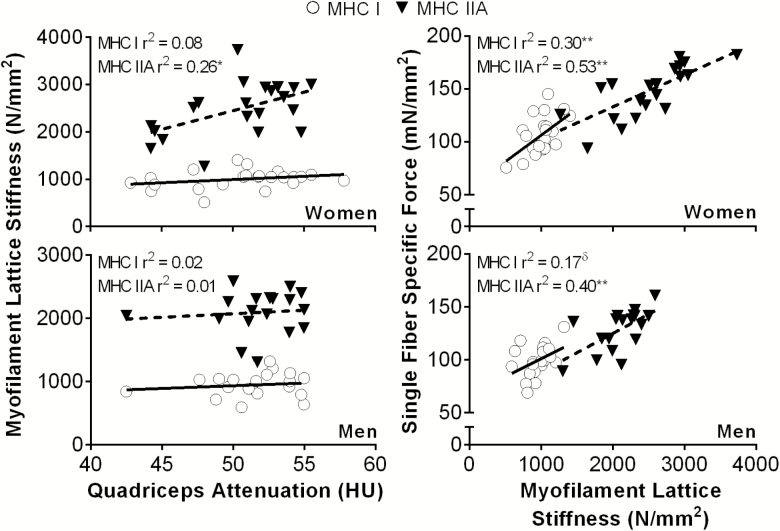
If adiposity impairs cellular function, we would predict effects on the underlying molecular determinants, such as myofilament and myosin–actin cross-bridge mechanic and kinetic properties. In keeping with this notion, in older women, myofilament lattice stiffness in MHC IIA fibers was reduced with lower quadriceps attenuation (Figure 2). Importantly, myofilament lattice stiffness is strongly related to specific force production in MHC I and IIA fibers in both sexes (Figure 2).
Figure 2.
Relationships between (left) myofilament lattice stiffness (parameter A from sinusoidal analysis) in myosin heavy chain (MHC) I and IIA single muscle fibers and intramuscular lipid content and (right) specific force and myofilament lattice stiffness in older men (n = 18) and women (n = 21). *p < .05; **p < .01; δp = .07.
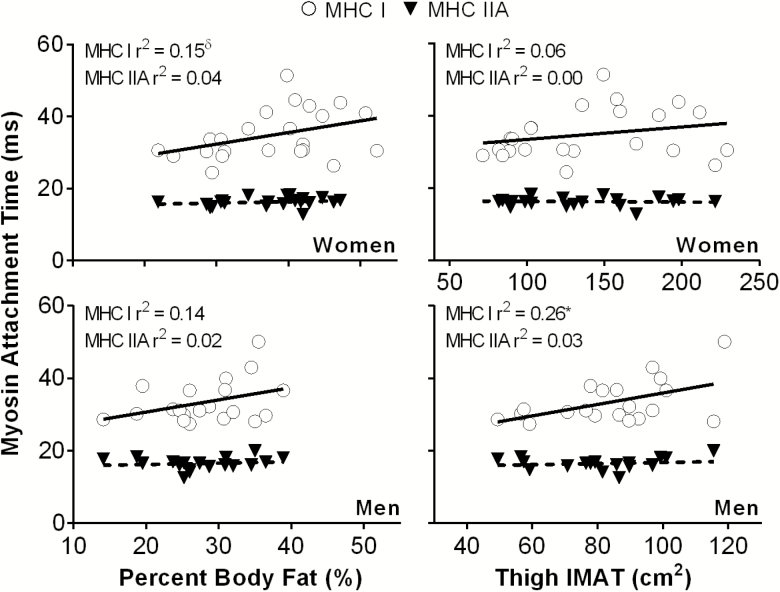
We also examined the relationship between cross-bridge kinetics and measures of whole-body and regional adiposity. Myosin attachment time was the only measure related to adiposity when men and women were examined as a single group; specifically, myosin attachment time in MHC I fibers was negatively related to quadriceps attenuation (r = −.33, p = .03) and positively related to percent body fat (r = .38, p = .01). When sex-specific correlations were performed, myosin attachment time (ton) in MHC I fibers was related to thigh intermuscular adipose tissue (r = .51, p = .02, Figure 3) in older men, and showed a trend toward being related to percent body fat (r = .39, p = .06, Figure 3) in older women. No additional relationships between molecular function and regional (quadriceps attenuation or thigh and quadriceps intermuscular adipose tissue) adiposity for either fiber type were found in men (p = .07–.97) or women (p = .08–.90).
Figure 3.
Relationships between myosin attachment time in myosin heavy chain (MHC) I and IIA single muscle fibers and adiposity in older men (n = 18) and women (n = 21). IMAT = intermuscular adipose tissue. *p < .05; δp = .06.
Relationships Between Thigh Composition and Whole-Body Adiposity
Relationships of molecular and cellular functional indices with local adiposity measures may simply reflect the fact that local fat tracks with whole-body adiposity. To begin to explore this possibility, we evaluated the relationship between local and total adiposity measures. Quadriceps attenuation was strongly correlated to percent body fat (r = −.77, p < .01) in women, but not men. Notably, thigh intermuscular adipose tissue was positively correlated with percent body fat in both men (r = .71, p < .01) and women (r = .84, p < .01), whereas quadriceps intermuscular adipose tissue only correlated with percent body fat in women (r = .47, p < .05). Thigh muscle size did not correlate (men: p = .97; women: p = .08) with increasing whole-body adiposity in either sex.
Partial Correlations
Considering the correlations between local and total adiposity measures, we sought to determine if the sex-specific relationships of inter- and intramuscular fat to molecular and cellular functional indices were independent of total adiposity. To accomplish this, we evaluated partial correlations between local fat indices and functional measures with statistical adjustment for percent body fat. The relationship between quadriceps attenuation and specific force in fibers from older women remained in MHC I (r = .42, p = .05), but not MHC IIA (r = .24, p = .31) fibers. In addition, associations between thigh intermuscular adipose tissue and cellular and molecular function were diminished following adjustment for percent body fat. Thigh intermuscular adipose tissue was no longer associated with specific force in MHC IIA fibers (r = −.09, p = .70) from older women nor with myosin attachment time in MHC I fibers from older men (r = .38, p = .11).
Relationships Between Intramyocellular Lipid and Contractile Function
Similar to relationships observed for other measures of body fat, the associations between intramyocellular lipid and cellular and molecular contractile function were sex-specific. In agreement with findings using measures of whole-body and regional adiposity, a greater number of lipid droplets from ORO was associated with longer myosin attachment times (r = .81, p = .05) in MHC I fibers from older men. Likewise, in older women, greater relative area occupied by lipid droplets from EM trended toward reduced specific force (r = −.41, p = .06) in MHC I fibers. Notably, values we obtained are similar to others performing ORO (10) and EM (30) in older adults.
Discussion
A growing body of evidence indicates that adiposity negatively affects in vivo skeletal muscle function in older adults (8–10), with recent evidence suggesting a possible influence of sex (11). Seminal work from the Health, Aging, and Body Composition study showed that lower muscle attenuation was independently associated with reduced specific strength (12) and incident mobility limitations (31) in older adults, and more recent work found relationships between quadriceps attenuation and knee extensor rate of torque development and peak torque in a large cohort of nearly 5,000 older adults (32). In this study, lower quadriceps attenuation was associated with reduced knee extensor torque and power, but only in older women, suggesting that adiposity may affect whole-muscle function in a sex-specific manner. Despite these observations, a mechanism by which lipid may impair cellular and molecular contractility is unclear.
To the best of our knowledge, only one study has examined the impact of fat deposition on in vitro skeletal muscle mechanics in older humans (10). Choi and colleagues (10) compared in vivo knee extensor function and in vitro single muscle fiber contractile properties between 13 normal-weight (BMI = 22 kg/m2) and 24 overweight/obese (BMI = 30 kg/m2) older men and women. Specific force of MHC I and IIA fibers was reduced in the obese group relative to normal-weight adults. Moreover, in a subgroup (five normal-weight and five overweight/obese) analysis, both single-fiber shortening velocity and normalized power were inversely associated with intramyocellular lipid content (lipid droplet area) in the obese group when all fiber types were pooled. Another subgroup analysis (nine overweight/obese) showed a negative relationship between quadriceps ultrasound echo intensity, a marker of skeletal muscle lipid content, and specific force of pooled MHC I and IIA fibers. Our results extend this work to make two novel observations: (a) intramuscular lipid content may have sex-specific effects on whole-muscle and cellular contractile function (Figure 1), and (b) identification of potential molecular mechanisms for these effects of fat (Figures 2 and 3).
Sex-Specific Associations Between Adiposity and Skeletal Muscle Function
Relationships between skeletal muscle function and adiposity were first examined with men and women combined as a single sample. Although we did observe some correlations at various anatomical levels, adiposity was not consistently related to muscle function in the pooled cohort. However, when we performed sex-specific analyses, a number of relationships between muscle function and whole-body, regional, and cellular adiposity were revealed. Notably, although quadriceps attenuation was similar between the sexes (Table 1), lower values were associated with reduced knee extensor torque and power (whole-muscle level) and specific force in MHC I and IIA muscle fibers (cellular level) among older women, but not men (Figure 1). We also observed a trend toward reduced specific force in MHC I fibers from older women with a greater relative area occupied by lipid droplets (measured via EM). In the context of age-related changes in body composition (1, 2), impaired muscle function in the presence of adiposity could manifest in a cyclical fashion, whereby lower physical performance results in decreased physical activity and/or exercise, ultimately leading to greater adiposity and more pronounced declines in muscle function. Thus, from a clinical standpoint, reducing adiposity may improve physical performance (eg, stair climbing, gait speed) through better skeletal muscle strength and power.
Potential Molecular Mechanisms for Reduced Single Fiber–Specific Force
Although the detrimental effects of body fat on whole-muscle function in older adults are becoming increasingly apparent (8–12, 32), knowledge of the mechanisms underlying how fat may affect contractility is limited (10). However, as cellular and molecular contractile properties contribute to whole-muscle strength and power (13), our current results suggest that fat could possibly impair skeletal muscle function by: (a) reducing myofilament lattice stiffness, and/or (b) slowing myosin–actin cross-bridge kinetics. Greater myofilament lattice stiffness increases maximal Ca2+-activated specific force, as shown in modeling work (33), the present study (Figure 2), and previous work by others (34). If adiposity decreases myofilament stiffness, which, in turn, will reduce force transmission, this could lead to lower single fiber–specific force. Regarding myosin–actin cross-bridge kinetics, we observed longer myosin attachment times in MHC I fibers with greater thigh intermuscular adipose tissue in older men, and a trend with greater percent body fat in older women. Likewise, a greater number of lipid droplets derived via ORO was associated with longer myosin attachment times in MHC I fibers from older men. As shortening velocity is inversely proportional to myosin attachment time (35), our observations may partially explain the correlation between reduced unloaded shortening velocity and number of lipid droplets in obese older adults observed by Choi and colleagues (10), providing a molecular basis for their results. As whole-muscle function is partly determined by contractile properties of the underlying muscle fibers (36), alterations in molecular and cellular function observed in this study may partially explain the previous observations of reduced whole-muscle strength and power with greater adiposity in older women (8–12). Of note, the association between quadriceps attenuation and specific force of MHC I fibers in older women remained after statistical control for whole-body adiposity, suggesting a possible unique effect of local fat stores on fiber contractility. Further mechanistic studies will be required to determine if these relationships reflect detrimental effects of local fat stores or an effect of greater overall adiposity.
Intermuscular Adipose Tissue
A number of studies suggest that age-related increases in intermuscular adipose tissue (3) may contribute to muscle dysfunction and mobility disability (37). We found that thigh intermuscular adipose tissue was associated with cellular (specific force in MHC IIA fibers in women) and molecular (myosin attachment time in MHC I fibers from men) muscle function. However, these relationships were no longer significant following adjustment for whole-body adiposity. Diminution of this relationship may reflect the fact that thigh intermuscular adipose tissue tracked closely with percent body fat in both sexes. Studies that investigate the independent effects of different fat depots (intramuscular lipid, intermuscular adipose tissue, and overall adiposity) on muscle function at various anatomical levels (molecular, cellular, and whole muscle) will be necessary to better understand how adiposity regulates muscle contractility.
How might local or total adiposity regulate contractility? One potential pathway is through the effects of excess adiposity to increase oxidative stress (38). Indeed, we have shown that chemical modifications that mimic oxidative modification of myofilaments can produce the phenotypes observed with increased adiposity in the current study, such as slowed cross-bridge kinetics (15), and others have shown that oxidants can reduce maximal force (39). Why such effects differ by sex, however, is uncertain, although recent evidence suggests sex-specific relationships between markers of oxidative stress and muscle strength in older adults, with stronger associations in women (40). One possible explanation is variation in sex hormone levels. Menopause is characterized by a decrease in ovarian hormone levels in women and these hormones may regulate skeletal muscle function. For instance, estradiol is an independent predictor of whole-muscle strength and power in pre- and postmenopausal women (41), but is not related to muscle strength in older men (42). Studies in rodents have shown that estradiol deficiency reduces myosin regulatory light chain phosphorylation (43), which regulates force in striated muscle (44). These preclinical results are in accordance with data from our laboratory in older women (14). Moreover, in humans, hormone replacement therapy increased specific force of MHC I and IIA fibers in postmenopausal monozygotic female twins compared to nonusers (45). Loss of the regulatory effects of estrogen on single fibers with menopause may allow for other factors, such as lipid content, to assert a more powerful effect on function in older women. In addition to our results, others have shown that sex affects the relationship between whole-body adiposity and physical function, such that older women are affected to a greater extent than men (18, 46). Thus, evidence continues to emerge that suggests adiposity influences muscle function of older adults in a sex-specific manner.
Limitations
Several limitations to our study need discussion. First, we cannot discern cause and effect from correlation analysis, as it is equally plausible that reduced muscle function could predispose individuals to increased adiposity, and ectopic fat deposition within skeletal muscles specifically. Further mechanistic studies are needed to discern a role for intramuscular fat and its sequelae in contractile function. Second, although altered myosin–actin cross-bridge kinetics and reduced specific force are plausible contributors to cellular and whole-muscle level dysfunction, other factors may play a role in obesity-related functional impairments. For example, adiposity can influence muscle fiber–type composition (29), specifically, promoting a more fast-twitch phenotype. In this study, however, we found no evidence of a phenotypic shift toward a faster fiber-type composition with greater whole-body or regional adiposity, suggesting that this would not mediate sex differences in whole-muscle/body function. Third, other factors that may be altered with adiposity, including systemic inflammation (47), neuromuscular activation (48), and bioactive lipid metabolites (eg, ceramide) (49), could partially explain the effects of obesity on muscle function. Finally, although we did measure intramyocellular lipid content using two approaches, we had a reduced sample size for ORO measurements (n = 12), which was similar to the study by Choi and colleagues (10), but limited our statistical power for sex-specific analyses. Nonetheless, we observed associations between intramyocellular lipid and cellular/molecular contractile function that were consistent with our primary results (eg, reduced specific force in MHC I fibers from older women), suggesting that adiposity may exert sex-specific effects on muscle function.
Conclusion
In conclusion, our results suggest relationships between thigh intramuscular lipid content and whole-muscle, cellular, and molecular contractile function in older adults, with stronger associations in women. As obesity reduces physical function among older adults (4, 5) and older women are at greater risk for obesity-related disability than men (50), understanding the cellular and molecular underpinnings of obesity-induced decrements in whole skeletal–muscle function, and the sex-specific nature of these relationships, may aid in developing interventions that forestall the onset of disability.
Funding
This work was supported by the National Institutes of Health (AG-031303 to M.S.M. and AG-033547 to M.J.T.).
Conflict of interest statement
None declared.
Acknowledgements
We thank all the volunteers who dedicated their valuable time to these studies.
References
- 1. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059 [DOI] [PubMed] [Google Scholar]
- 2. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Delmonico MJ, Harris TB, Visser M, et al. ; Health, Aging, and Body Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woo J, Leung J, Kwok T. BMI, body composition, and physical functioning in older adults. Obesity (Silver Spring). 2007;15:1886–1894. doi: 10.1038/oby.2007.223 [DOI] [PubMed] [Google Scholar]
- 5. Riebe D, Blissmer BJ, Greaney ML, Garber CE, Lees FD, Clark PG. The relationship between obesity, physical activity, and physical function in older adults. J Aging Health. 2009;21:1159–1178. doi: 10.1177/0898264309350076 [DOI] [PubMed] [Google Scholar]
- 6. Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;219:1–8. [PubMed] [Google Scholar]
- 7. Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more?J Gerontol A Biol Sci Med Sci. 2003;58:728–733. doi: 10.1093/gerona/58.8.M728 [DOI] [PubMed] [Google Scholar]
- 8. Newman AB, Haggerty CL, Goodpaster B, et al. ; Health Aging and Body Composition Research Group Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x [DOI] [PubMed] [Google Scholar]
- 9. Brady AO, Straight CR, Schmidt MD, Evans EM. Impact of body mass index on the relationship between muscle quality and physical function in older women. J Nutr Health Aging. 2014;18:378–382. doi: 10.1007/s12603-013-0421-0 [DOI] [PubMed] [Google Scholar]
- 10. Choi SJ, Files DC, Zhang T, et al. Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. J Gerontol A Biol Sci Med Sci. 2016;71:557–564. doi: 10.1093/gerona/glv169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Culvenor AG, Felson DT, Niu J, et al. Thigh muscle specific-strength and the risk of incident knee osteoarthritis: the influence of sex and greater body mass index. Arthritis Care Res (Hoboken). 2017;69:1266–1270. doi: 10.1002/acr.23182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the health ABC study. J Appl Physiol. (1985). 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157 [DOI] [PubMed] [Google Scholar]
- 13. Miller MS, Toth MJ. Myofilament protein alterations promote physical disability in aging and disease. Exerc Sport Sci Rev. 2013;41:93–99. doi: 10.1097/JES.0b013e31828bbcd8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller MS, Bedrin NG, Callahan DM, et al. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol. (1985). 2013;115:1004–1014. doi: 10.1152/japplphysiol.00563.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Callahan DM, Miller MS, Sweeny AP, et al. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J Physiol. 2014;592:4555–4573. doi: 10.1113/jphysiol.2014.279034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Callahan DM, Bedrin NG, Subramanian M, et al. Age-related structural alterations in human skeletal muscle fibers and mitochondria are sex specific: relationship to single-fiber function. J Appl Physiol. (1985). 2014;116:1582–1592. doi: 10.1152/japplphysiol.01362.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tseng LA, Delmonico MJ, Visser M, et al. Body composition explains sex differential in physical performance among older adults. J Gerontol A Biol Sci Med Sci. 2014;69:93–100. doi: 10.1093/gerona/glt027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valentine RJ, Misic MM, Rosengren KS, Woods JA, Evans EM. Sex impacts the relation between body composition and physical function in older adults. Menopause. 2009;16:518–523. doi: 10.1097/gme.0b013e31818c931f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Callahan DM, Tourville TW, Miller MS, et al. Chronic disuse and skeletal muscle structure in older adults: sex-specific differences and relationships to contractile function. Am J Physiol Cell Physiol. 2015;308:C932–C943. doi: 10.1152/ajpcell.00014.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller MS, Callahan DM, Tourville TW, et al. Moderate-intensity resistance exercise alters skeletal muscle molecular and cellular structure and function in inactive older adults with knee osteoarthritis. J Appl Physiol (1985). 2017;122:775–787. doi: 10.1152/japplphysiol.00830.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985). 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104 [DOI] [PubMed] [Google Scholar]
- 22. Miller MS, VanBuren P, LeWinter MM, et al. Chronic heart failure decreases cross-bridge kinetics in single skeletal muscle fibres from humans. J Physiol. 2010;588(Pt 20):4039–4053. doi: 10.1113/jphysiol.2010.191957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mulieri LA, Barnes W, Leavitt BJ, et al. Alterations of myocardial dynamic stiffness implicating abnormal crossbridge function in human mitral regurgitation heart failure. Circ Res. 2002;90:66–72. [DOI] [PubMed] [Google Scholar]
- 24. Palmer BM, Suzuki T, Wang Y, Barnes WD, Miller MS, Maughan DW. Two-state model of acto-myosin attachment-detachment predicts C-process of sinusoidal analysis. Biophys J. 2007;93:760–769. doi: 10.1529/biophysj.106.101626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao Y, Kawai M. The effect of the lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. II. Elementary steps affected by the spacing change. Biophys J. 1993;64:197–210. doi: 10.1016/S0006-3495(93)81357-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001;116:63–68. doi: 10.1007/s004180100297 [DOI] [PubMed] [Google Scholar]
- 27. Miller MS, Vanburen P, Lewinter MM, et al. Mechanisms underlying skeletal muscle weakness in human heart failure: alterations in single fiber myosin protein content and function. Circ Heart Fail. 2009;2:700–706. doi: 10.1161/CIRCHEARTFAILURE.109.876433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Acevedo LM, Raya AI, Rios R, Aguilera-Tejero E, Rivero JL. Obesity-induced discrepancy between contractile and metabolic phenotypes in slow- and fast-twitch skeletal muscles of female obese Zucker rats. J Appl Physiol (1985). 2017;123:249–259. doi: 10.1152/japplphysiol.00282.2017 [DOI] [PubMed] [Google Scholar]
- 29. Tanner CJ, Barakat HA, Dohm GL, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282:E1191–E1196. doi: 10.1152/ajpendo.00416.2001 [DOI] [PubMed] [Google Scholar]
- 30. Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA. The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biol Sci Med Sci. 2010;65:119–128. doi: 10.1093/gerona/glp179. [DOI] [PubMed] [Google Scholar]
- 31. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324 [DOI] [PubMed] [Google Scholar]
- 32. Frank-Wilson AW, Chalhoub D, Figueiredo P, et al. ; AGES-Reykjavik Study Associations of quadriceps torque properties with muscle size, attenuation, and intramuscular adipose tissue in older adults. J Gerontol A Biol Sci Med Sci. 2018;73:931–938. doi: 10.1093/gerona/glx262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tanner BC, Daniel TL, Regnier M. Sarcomere lattice geometry influences cooperative myosin binding in muscle. PLoS Comput Biol. 2007;3:e115. doi: 10.1371/journal.pcbi.0030115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci. 2007;62:375–381. doi: 10.1093/gerona/62.4.375 [DOI] [PubMed] [Google Scholar]
- 35. Piazzesi G, Reconditi M, Linari M, et al. Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell. 2007;131:784–795. doi: 10.1016/j.cell.2007.09.045 [DOI] [PubMed] [Google Scholar]
- 36. Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol. 2000;73:195–262. doi: 10.1016/S0079-6107(00)00006-7 [DOI] [PubMed] [Google Scholar]
- 37. Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014;2014:309570. doi: 10.1155/2014/309570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond). 2006;30:400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 39. Dutka TL, Mollica JP, Lamb GD. Differential effects of peroxynitrite on contractile protein properties in fast- and slow-twitch skeletal muscle fibers of rat. J Appl Physiol (1985). 2011;110:705–716. doi: 10.1152/japplphysiol.00739.2010 [DOI] [PubMed] [Google Scholar]
- 40. Carru C, Da Boit M, Paliogiannis P, et al. Markers of oxidative stress, skeletal muscle mass and function, and their responses to resistance exercise training in older adults. Exp Gerontol. 2018;103:101–106. doi: 10.1016/j.exger.2017.12.024 [DOI] [PubMed] [Google Scholar]
- 41. Pöllänen E, Kangas R, Horttanainen M, et al. Intramuscular sex steroid hormones are associated with skeletal muscle strength and power in women with different hormonal status. Aging Cell. 2015;14:236–248. doi: 10.1111/acel.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–3282. doi: 10.1210/jcem.85.9.6825 [DOI] [PubMed] [Google Scholar]
- 43. Lai S, Collins BC, Colson BA, Kararigas G, Lowe DA. Estradiol modulates myosin regulatory light chain phosphorylation and contractility in skeletal muscle of female mice. Am J Physiol Endocrinol Metab. 2016;310:E724–E733. doi: 10.1152/ajpendo.00439.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stull JT, Kamm KE, Vandenboom R. Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle. Arch Biochem Biophys. 2011;510:120–128. doi: 10.1016/j.abb.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qaisar R, Renaud G, Hedstrom Y, et al. Hormone replacement therapy improves contractile function and myonuclear organization of single muscle fibres from postmenopausal monozygotic female twin pairs. J Physiol. 2013;591:2333–2344. doi: 10.1113/jphysiol.2012.250092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fragala MS, Clark MH, Walsh SJ, et al. Gender differences in anthropometric predictors of physical performance in older adults. Gend Med. 2012;9:445–456. doi: 10.1016/j.genm.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schrager MA, Metter EJ, Simonsick E, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol (1985). 2007;102:919–925. doi: 10.1152/japplphysiol.00627.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yoshida Y, Marcus RL, Lastayo PC. Intramuscular adipose tissue and central activation in older adults. Muscle Nerve. 2012;46:813–816. doi: 10.1002/mus.23506 [DOI] [PubMed] [Google Scholar]
- 49. Rivas DA, McDonald DJ, Rice NP, Haran PH, Dolnikowski GG, Fielding RA. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. Am J Physiol Regul Integr Comp Physiol. 2016;310:R561–R569. doi: 10.1152/ajpregu.00198.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11:568–579. doi: 10.1111/j.1467-789X.2009.00703.x [DOI] [PubMed] [Google Scholar]