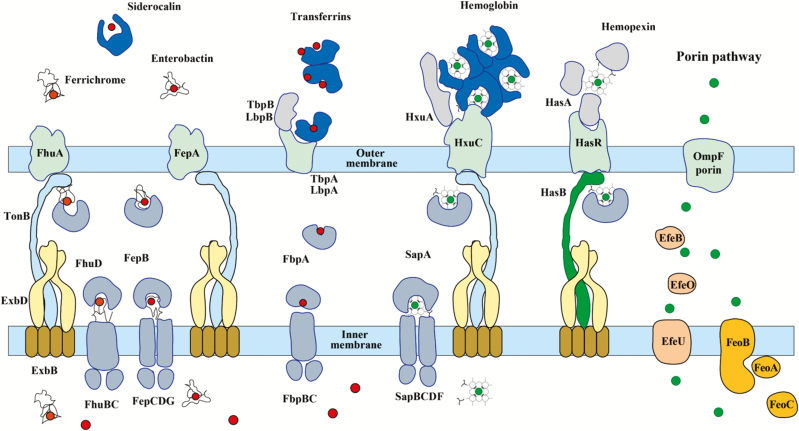
Figure 1.
Iron transporters in gram-negative bacteria and metal availability in the host during infection. In a healthy individual, ferric iron (Fe3+; red circles) circulates bound by transferrin in the blood, and ferrous iron (Fe2+; green circles) is complexed in heme, which is bound by hemoglobin within red blood cells but can be released by hemolysis during infection. Free Fe2+ is uncommon; however, when available, it enters through the general porin pathway. Free heme is scavenged by hemopexin. Secreted bacterial siderophores remove iron from transferrins and ferritin, and the siderophore–iron complexes are bound by cognate receptors at the bacterial surface. Similarly, secreted hemophores such as HasA and HxuC can remove heme from hemoglobin and hemopexin. Enterobacteria also possess outer membrane receptors for heme. In Neisseria, iron transferrins are bound by outer membrane receptors comprising 2 subunits (eg, LbpA and LbpB for lactoferrin) and forced to release 1 of the bound iron ions. Catecholate-mediated iron acquisition (eg, by enterobactin) can be inhibited by the innate immune protein lipocalin-2 (siderocalin or neutrophil gelatinase-associated lipocalin), which binds and sequesters catechols.