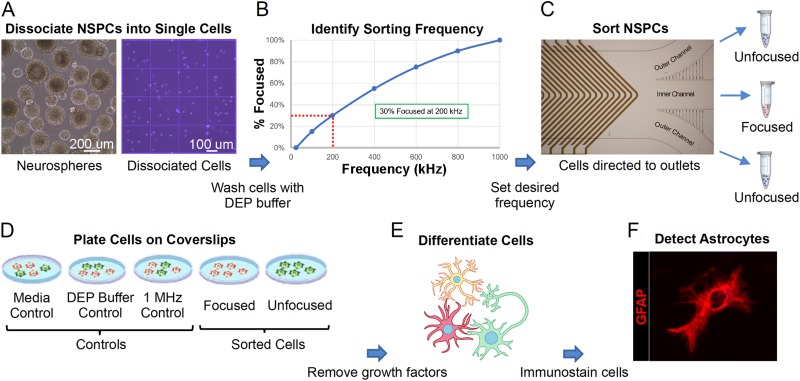
FIG. 5.
Experimental strategy for sorting mouse NSPCs with the HOAPES device. (a) Mouse NSPCs grow in suspension and form clusters of cells known as neurospheres (left). Neurospheres were dissociated to form single cells (right) and cells washed with the DEP buffer. (b) Cells in the DEP buffer were loaded into the HOAPES device, and the percentage of cells focused to the inner channel quantified across a range of frequencies. The resultant focusing curve (shown here as a schematic) was used to determine a sorting frequency targeting ∼30% of the cells. (c) Mouse NSPCs were sorted in the HOAPES device at the determined frequency and two populations of cells collected: those focused to the inner channel and those that remained unfocused and tracked to the outer channels. The unfocused cells from the two outer channels were pooled for further analysis. (d) Sorted cells (focused and unfocused) and controls (media, DEP buffer, and 1 MHz) were plated on glass coverslips for cellular differentiation and further analysis. (e) Cell differentiation was induced by the removal of growth factors from the culture media. Cells were differentiated for 3 days to allow formation of astrocytes. (f) Astrocytes in the differentiated cell samples were detected by immunostaining with antibodies that detect the astrocyte marker glial fibrillary acidic protein (GFAP, red). The image shows a differentiated astrocyte.