Figure 5.
FT–PC Interaction Affects Regulation of Effector Genes
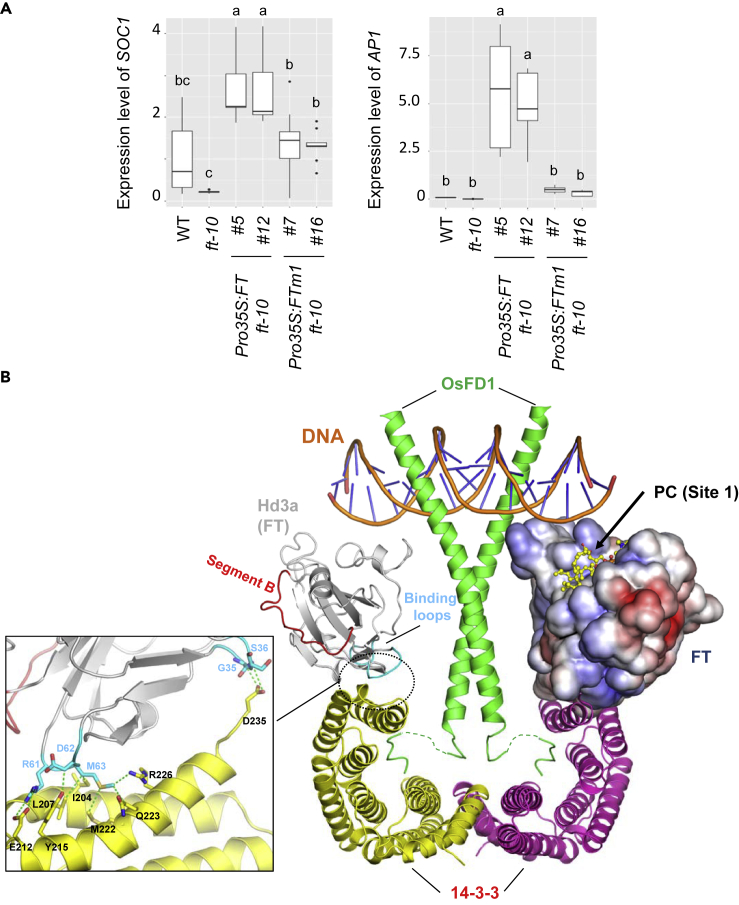
(A) The expression of SOC1 and AP1 in shoot apices of 7- and 14-day-old seedlings, respectively. Data are mean ± SD of three biological replicates. Different letters indicate significant difference at p < 0.05 determined by one-way ANOVA with Tukey's post-test.
(B) A proposed structural model of florigen activation complex (FAC) comprising two Hd3a (FT homologue) molecules, a 14-3-3 dimer (yellow and magenta), bZIP domains of OsFD1 (green), and DNA (orange). The proposed model is based on the crystal structure of Hd3a in complex with the 14-3-3 protein and the C-terminal region of OsFD1 peptide (PDB: 3AXY). A present structure of FT is superposed onto the right-side Hd3a, as shown in surface representation colored by electrostatic surface potential. A complex model of the bZIP domain of OsFD1 and DNA was built based on the complex structure of the mouse CREB bZIP region (residues 285–339) and C-box DNA (Taoka et al., 2011; PDB: 1DH3). The predicted bound PC molecule is shown in a ball-and-stick model on the right-side FT molecule. Segment B and the 34–37 and 61–64 binding loops of Hd3a (the 32–35 and 59–62 loops in FT) are shown in red and cyan, respectively, on the left-side Hd3a molecule. The interaction between the binding loops of Hd3a and 14-3-3 protein is highlighted in the inset. Van der Waals contacts between them are shown by green dashed lines. Other interactions at the interface between Hd3a and 14-3-3 are omitted for clarity.