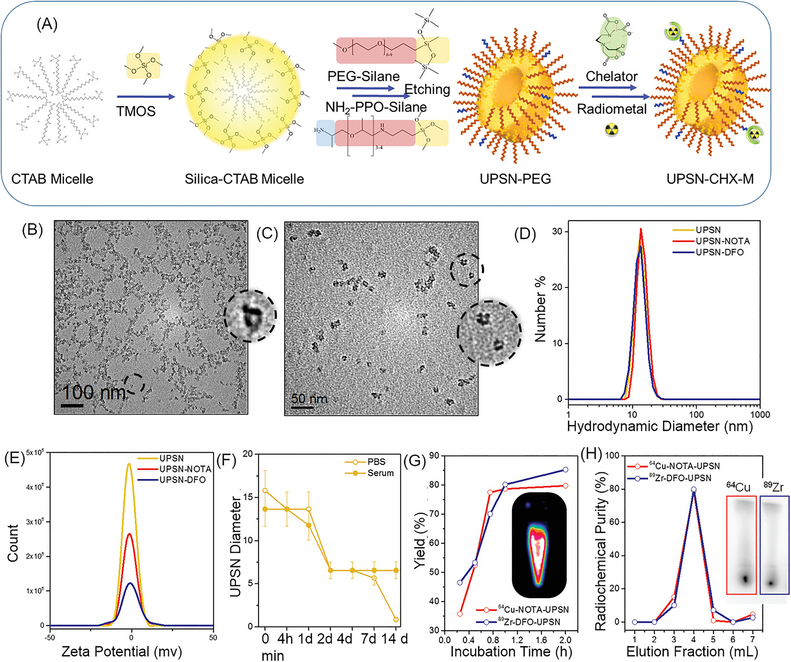
Figure 1. Synthesis and Characterization.
(A) Schematic of the synthesis strategy and surface modification of UPSNs. Rapidly hydrolyzing silica precursor TMOS is condensed on CTAB (surfactant) micelles, followed by arrest of nanoparticle growth by addition of PEG-silane (capping agent). NH2-PPO-silane is added to impart functional amine groups for further conjugation of chelator (CHX) for radiolabeling or fluorescent dyes for in vivo studies. TEM images of (B) UPSN and (C) UPSN-CHX. Insets show a magnified image of an individual UPSN with fully-formed single pores in the center. (D) Hydrodynamic diameters and (E) zeta potential measurements of UPSN and UPSN-CHX in water/PBS. (F) Change in hydrodynamic diameters of UPSN immersed in PBS or serum, over a period of 2 weeks. (G) Radiolabeling yield and (H) radiochemical purity of 64Cu-NOTA-UPSN and 89Zr-DFO-UPSN. Inset in (G) depicts PET image of 64Cu-NOTA-UPSN sample. Inset in (H) represents thin layer chromatographs (TLC) of 64Cu-NOTA-UPSN and 89Zr-DFO-UPSN depicting high radiochemical yield and purity with negligible migration of free isotope to the solvent front.