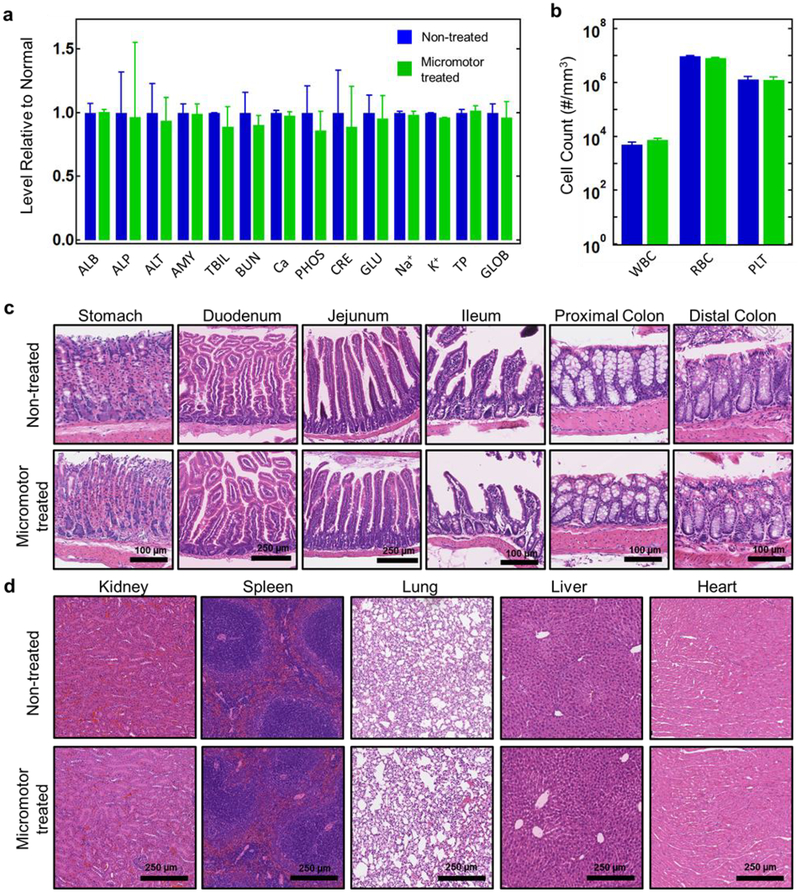
Figure 4.
In vivo evaluation of Fe/Se-loaded micromotor toxicity. a, Comprehensive blood chemistry panel taken from non-treated mice and mice treated for 30 days with Fe/Se-loaded micromotors (n = 3). ALB: albumin; ALP: alkaline phosphatase; ALT: alanine transaminase; AMY: amylase; TBIL: total bilirubin; BUN: blood urea nitrogen; Ca: calcium; PHOS: phosphorus; CRE: creatinine; GLU: glucose; Na+: sodium; K+: potassium; TP: total protein; GLOB: globulin (calculated). b, Count of various blood cells taken from non-treated mice and mice treated for 30 days with Fe/Se-loaded micromotors (n = 3). WBC: white blood cells; RBC: red blood cells; PLT: platelets. c, Hematoxylin and eosin (H&E) staining of representative histology sections from different portions of the GI tract from non-treated mice and mice treated for 30 days with Fe/Se-loaded micromotors. d, H&E staining of representative histology sections of major organs from non-treated mice and mice treated for 30 days with Fe/Se-loaded micromotors. Error bars are defined as mean ± SD.