Abstract
Background.
Alcohol, depression, and intimate partner violence (IPV) are endemic in sub-Saharan Africa. This article examines whether and how these conditions affect mothers living with HIV (MLH), compared to mothers without HIV (MWOH). In particular, we assess the influence of these comorbidities on engagement in HIV care and adherence to antiretroviral therapies (ARV) among MLH. Data on maternal HIV care are typically based on clinic samples, with substantial loss to follow-up. This study fills that gap by including all mothers in specified areas.
Setting.
A cohort study examines MLH in Cape Town, South Africa recruited in pregnancy and followed repeatedly for five years, compared to MWOH.
Methods.
Almost all (98%) pregnant women in 12 neighborhoods (N=594) were recruited in pregnancy. Mothers and children were reassessed five times over five years with high retention rates at each of the six assessments, from 98.7% at two weeks to 82.8% at 5 years post-birth. MLH’s uptake and adherence to HIV care was evaluated over time associated with maternal comorbidities of alcohol use, depressed mood, and IPV using mixed effects logistic regression.
Results.
MLH have fewer resources (income, food, education) and are more likely to face challenges from alcohol, depression, and having seropositive partners over time than MWOH. Only 22.6% of MLH were consistently engaged in HIV care from six months to five years post-birth. At five years, 86.7% self-reported engaged in HIV care, 76.9% were receiving ARVs and 87% of those on ARV reported consistent ARV adherence. However, data on viral suppression are unavailable. Alcohol use, but not depressed mood or IPV, was significantly related to reduced uptake of HIV care and adherence to ARV over time.
Conclusions.
Adherence to lifelong ARV by MLH requires a combination of structural and behaviorally-focused interventions. Alcohol abuse is not typically addressed in low and middle-income countries, but is critical to support MLH.
Keywords: Alcohol Use, HIV, Depression, Intimate Partner Violence, Adherence
INTRODUCTION
Pregnant women living with HIV require engagement in HIV care and adherence to antiretroviral treatment (ARV) in order prevent mother-to-child transmission (PMTCT). About 70% of mothers living with HIV (MLH) in sub-Saharan Africa are identified during pregnancy and the majority are linked to services [1]. Unfortunately, many studies have reported high loss to follow-up of pregnant MLH; about 33% to 50% of women drop out of PMTCT during pregnancy [2,3], and another 20% to 33% are lost by 6 months post birth [4–7]. Furthermore, many MLH who remain on ARV are often not consistently adherent. Recent studies of pregnant and post-partum women found 13% of MLH already on ARV have unsuppressed viral loads at their first antenatal care visit, 50% are not adherent at 6 months, 37% of MLH will have at least one episode of viremia (viral load > 1000), and 13% will be virally unsuppressed (two consecutive episodes of viremia) within 12 months of ARV initiation [8–11]. With these rates of drop-out, poor adherence, and viremia breakthrough, many countries in sub-Saharan Africa are unlikely to achieve the Joint United Nations Programme on HIV/AIDS goals that by 2020 90% of all people living with HIV will know their status; 90% of all people with diagnosed HIV infection will receive sustained antiretroviral therapy; and 90% of all people receiving antiretroviral therapy will have viral suppression [12].
Therefore, understanding the barriers MLH face when caring for themselves is a high priority. For example, it is known that alcohol use accelerates HIV disease progression through both immunological and behavioral mechanisms [13]. Depression is associated with worse HIV adherence and viral suppression [14], and most new diagnoses for HIV and depression are made during the perinatal period, making this a time of increased risk [1, 15]. Intimate partner violence (IPV) affects about 30% of women globally and is associated with a higher risk of acquiring HIV [16]. One study in South Africa estimated that 12% of new HIV infections could be avoided if women did not experience more than one episode of IPV [17].
South Africa experiences syndemic comorbidities of HIV, alcohol use, depression, and IPV [18]. There is substantial evidence that these maternal comorbidities reduce uptake and adherence to ARV in low and middle-income countries [19–22]. Yet, most of these studies are based on cross-sectional analyses of clinic samples. There are few longitudinal samples of MLH and the impact of these comorbidities on HIV care uptake and adherence over time. The current study aims to help fill this gap.
To understand the rate of comorbidities among MLH, it is critical to concurrently compare the outcomes of mothers without HIV (MWOH) in the same communities. With such comparison, we can disentangle whether alcohol, depression and IPV affect MLH and then, by association, their engagement, retention, and adherence to HIV care. We hypothesize that the comorbid challenges of alcohol, depression, and IPV will reduce the ability of MLH to seek HIV care, thus playing a moderating role in long-term outcomes.
METHODS
Ethics Statement
The Institutional Review Boards of (BLIND FOR REVIEW) and (BLIND FOR REVIEW) approved the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Setting
Cape Town has a network of townships with both formal homes and informal shacks. Most families (70%) earn from 0-800 South African Rand (0-60 USD) per month. Townships typically have tarred roads, mast lighting, central water pumps, water-borne sewage services, and some access to electricity and telephones. We identified 24 neighborhoods of similar size (450-600 households) that were within five kilometers of health clinics, had five to seven alcohol bars, and were non-contiguous and/or separated by natural barriers (e.g., a highway). Half of these neighborhoods were randomized to a control condition of a RCT and received standard care [23].
South African national recommendations for PMTCT changed during the recruitment time period. From 2008, they were: 1) lifelong triple drug ARV for MLH with CD4 counts ≤ 200 and single dose nevirapine (sdNVP) followed by 7 days zidovudine (AZT) for their infants; 2) AZT during pregnancy and sdNVP added during labor for MLH with CD4 counts >200 and sdNVP followed by 7 days AZT for their infants; 3) for MLH who received less than 4 weeks of any ARV treatment during pregnancy, infants receive sdNVP and 28 days of AZT [24]. From 2010 they were: 1) lifelong triple drug ARV for MLH with CD4 counts ≤ 350 and 6 weeks NVP for their infants; 2) AZT during pregnancy, nevirapine (NVP) added during labor, and single dose dual ARV after delivery for MLH with CD4 counts >350 and single dose NVP followed by 6 weeks minimum, continued as long as breastfeeding occurs, AZT for their infants [25].
Participants
All mothers from the 12 neighborhoods in the standard care condition are included in this report, both MLH and MWOH. Recruiters approached households in each neighborhood repeatedly from May 2009 to September 2010 and recruited 98% of pregnant women in a study of family well-being (n=594).
Assessments
Township women were trained as interviewers and used mobile phones to enter data from questionnaires, observational measures, and performance tasks. Mothers were transported to a nearby research site for the assessments. Mothers were assessed during pregnancy, and 98.7% were reassessed at two weeks post-birth, 93.2% at 6 months, 91.7% at 18 months, 84.7% at 36 months, and 82.8% at 60 months post-birth. Seventy percent (70%) of mothers/children completed all assessments. Over five years 5 mothers and 55 infants died; dyads were removed at death of either mother or child.
Measures
Maternal demographic characteristics include age, highest grade of school completed, employment status, partnership status, household income, type of housing, and the presence of water on the property (or not), flush toilets, and electricity on site. Food insecurity was assessed using one item (“How many days in the past week have you (i.e. mother, child, or both) gone hungry?”) from the Household Food Insecurity Access Scale (HFIAS). This item has been found to be highly correlated with the nine-item scale used to distinguish food insecure from food secure households across different cultural contexts [26]. We also asked women about their recent sexual partnerships, their knowledge of the partners’ HIV status, and the likelihood that they would ask them to test for HIV.
Maternal Comorbidities.
Alcohol use.
Women reported on any alcohol use at each assessment. At baseline, alcohol use prior to discovery of pregnancy was reported; at the post-birth assessment, alcohol use in the month prior to birth was reported; at six months, alcohol use since the baby was born was reported; and at the remaining assessments, alcohol use in the month prior to the assessment was reported. Problematic drinking, was constructed based on whether the participant experienced heavy episodic drinking (four or more drinks in a single day) at least once a month over a specified timeframe, and responded yes to at least one of the following three questions: (1) Have close friends or relatives worried or complained about your drinking?; (2) Do you sometimes take a drink in the morning when you first get up?; (3) Has a friend or family member ever told you about things you said or did while you were drinking that you could not remember [27]?
Depressive symptoms.
At each assessment, the Edinburgh Postnatal Depression Scale (EPDS) [28] was administered. A cut-off of ≥ 13 was used to indicate depressed mood [29].
Intimate partner violence was captured at each assessment time point and asked whether the woman had been slapped, pushed or shoved, and/or threatened with a weapon by a current partner in the past 12 months.
Maternal HIV Care.
At each assessment, mothers self-reported their HIV status. Maternal self-reports of HIV status were validated in three ways. First, maternal HIV status was reported on children’s government-issued Road-to-Health Card. These cards were reviewed at each assessment. Second, 62% of all mothers had additional children over the next 5 years (range 1-8 additional children). At each pregnancy, more than 98% of mothers were retested for HIV and again, the results are on the Road-to-Health card. Finally, at a 96-month assessment, we administrated rapid HIV tests. The correlation between self-report and the rapid HIV test was r=0.84. There were only 36 cases in which mothers self-reported that they were HIV seronegative, but tested seropositive on the rapid HIV test (6% inaccurate reports).
Additionally, among MLH, we measured engagement in HIV care, receipt of ARV, and ARV adherence. Based on self-report, mothers were considered engaged in HIV care if they reported having been to the clinic since the last assessment and/or currently being on ARVs, while ARV receipt was a “yes” or “no” response at each assessment at 6 months and on. MLH were considered adherent to the prescribed HIV regimen if they reported taking ARVs every day for the last seven days starting from the 18-month assessment to 5 years.
Data Analysis
We examined potential selection effects related to participant loss-to-follow-up at the five year assessment by comparing baseline characteristics of mothers who did not follow up (n=92) to those who completed the five year assessment (n=443).
For the purpose of these analyses, MLH’s HIV status was treated as a time-varying measurement since a mother could report a new infection at any follow-up assessment. However, once identified as positive, mothers’ HIV status was not allowed to change at later assessments. Differences in MLH and MWOH groups were assessed using t-tests for continuous variables and chi-square tests for discrete variables.
Longitudinal models were used to compare MLH and MWOH for maternal comorbidities. Mixed-effects logistic regression models were used to analyze discrete outcomes. Multivariable longitudinal models for maternal comorbidities assessed at multiple time points included fixed effects for mother’s HIV status, time point, and an interaction between mother’s HIV status and time point.
Regression models accounted for repeated subject measures within neighborhoods (i.e., nested design nature) and included random intercepts for neighborhood, as well as for the mother participant, where appropriate. For outcomes where model convergence was not achieved, we removed the neighborhood random effect and included neighborhood as a fixed effect in the model. Random slopes for time were assessed and if needed, were included in the longitudinal models in addition to the random intercepts and fixed effects. Appropriate covariance structure was then selected after comparison of models using likelihood ratio test (LRT) and Akaike information criterion (AIC) or Bayesian information criterion (BIC). In particular, we initially fed our model with the most complex covariance structure (e.g. unstructured); where applicable conducted LRT to test other candidate covariance structures (e.g. exchangeable, independent, identity) against the more complex model to see whether simpler model is adequate, and compared AIC/BIC between models, where the lower AIC/BIC indicates better fit to the data.
We examined mean differences between MLH and MWOH using the mean estimates on a measure at each follow-up point and the estimate on a measure at the baseline assessment, i.e. [(average of all MLH follow-up estimates) minus (MLH baseline estimate)] – [(average of all MWOH follow-up estimates) minus (MWOH baseline estimate)]. Missing data were assumed missing at random; thus, all analyses were performed on complete cases. We used all available observations up to the point where mothers died or were loss-to-follow-up. We used SAS 9.4 and Stata® SE software version 14.2. Regression analyses were performed using the -mixed- and -melogit- commands in Stata.
RESULTS
Mothers who were lost to follow-up at the five year assessment (n=92) were more educated, were less likely to live in formal housing or to have children who were hungry in the past week, less likely to have a prior child, were less likely to receive HIV results, and more likely to have knowledge of partner HIV status than those mothers who were retained at five years (n=443). HIV status was not associated with loss-to-follow-up (Results are available as a supplementary online table).
Outcomes of MLH and MWOH
A full report of baseline characteristics of MLH and MWOH can be found in Table 1. Ten mothers did not disclose their HIV status and were not included in the analyses. At recruitment, 26% of women were MLH. Over the course of five years, 26 additional mothers acquired HIV; five were identified at 18 months, six at 36 months, and 15 at 60 months. These mothers were included in baseline analysis. There were 11 HIV+ children identified over the 5 years. During the study, < 1.0% (n=5/584) of the mothers had died; 3 were seropositive and 2 seronegative.
Table 1.
Baseline characteristics of mothers living with HIV (MLH; n=179) and mothers without HIV (MWOH; n=405), as well as the overall sample
| MLH (N=179) | MWOH (N=405) | Total (N=584) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Demographic characteristics | ||||||
| Age, mean (SD)* | 27.0 | 5.2 | 26.0 | 5.7 | 26.3 | 5.6 |
| Highest education level (years), mean (SD)* | 10.0 | 1.8* | 10.4 | 1.8 | 10.3 | 1.8 |
| Currently employed | 25 | 14.0 | 78 | 19.3 | 103 | 17.6 |
| Married or lives with partner | 94 | 52.5 | 224 | 55.3 | 318 | 54.5 |
| Monthly household income > 2000 Rand* | 71 | 40.3* | 204 | 51.6 | 275 | 48.2 |
| Formal housing | 54 | 30.2 | 136 | 33.6 | 190 | 32.5 |
| Water on site | 94 | 52.5 | 227 | 56.0 | 321 | 55.0 |
| Flush toilet on site | 102 | 57.0 | 235 | 58.0 | 337 | 57.7 |
| Electricity on site | 159 | 88.8 | 375 | 92.6 | 534 | 91.4 |
| Mother hungry past week* | 103 | 57.5* | 190 | 46.9 | 293 | 50.2 |
| Children hungry past week | 62 | 34.6 | 118 | 29.1 | 180 | 30.8 |
| Partnerships | ||||||
| Reported a recent sexual partnera | 156 | 87.2 | 356 | 87.9 | 512 | 87.7 |
| Knowledge of recent partner’s HIV status | * | |||||
| Partner HIV+ | 50 | 32.1 | 0 | 0.0 | 50 | 9.8 |
| Partner HIV- | 34 | 21.8 | 257 | 72.2 | 291 | 56.8 |
| Partner serostatus unknown | 72 | 46.2 | 99 | 27.8 | 171 | 33.4 |
| Requested partner to take HIV test | 104 | 85.2 | 246 | 82.6 | 350 | 83.3 |
| Alcohol | ||||||
| Alcohol use in the month prior to pregnancy discovery | 50 | 32.1* | 77 | 23.1 | 127 | 25.9 |
| Problematic drinking in the month prior to pregnancy discovery | 43 | 27.6* | 57 | 17.1 | 100 | 20.4 |
| Alcohol use after pregnancy discovery | 17 | 11.0 | 32 | 9.6 | 49 | 10.0 |
| Problematic drinking after pregnancy discovery | 8 | 5.2 | 16 | 4.8 | 24 | 4.9 |
| Drank any alcohol, anytime during pregnancy | 57 | 31.8* | 95 | 23.5 | 152 | 26.0 |
| Depression | ||||||
| Edinburgh Postnatal Depression Scale (EPDS), mean (SD) | 11.4 | 7.3* | 9.9 | 7.0 | 10.3 | 7.1 |
| EPDS ≥ 13 | 71 | 39.7* | 118 | 29.1 | 189 | 32.4 |
| Intimate Partner Violence | ||||||
| Woman reports being slapped, pushed, shoved, and/or threatened with a weapon by a current partner in the past 12 months. | 69 | 38.6 | 144 | 35.6 | 213 | 36.5 |
“Recent” refers to the last three months
p < 0.05 (t-tests for continuous variable or X2 tests for discrete variables)
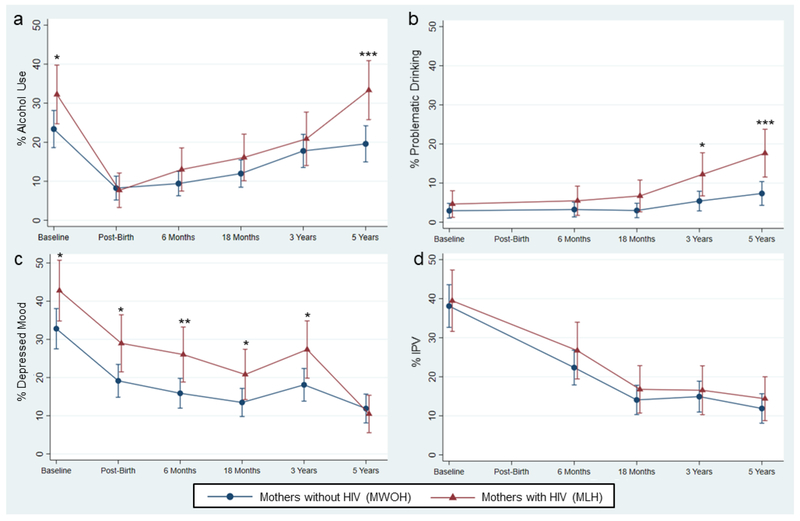
MLH were no more likely to die compared to MWOH. We display alcohol use, depressed mood, and IPV in MLH and MWOH at each time assessment from baseline to 60 months on Figure 1. Alcohol was used by 25% of mothers prior to recognizing that they were pregnant and reduced for most women after recognizing that they are pregnant. Alcohol use decreased substantially after childbirth and slowly increased over time until reaching pre-pregnancy levels at 5 years. Any use of alcohol was significantly different between MLH and MWOH at baseline (32.2% vs. 23.4%, p = 0.04) and 60 months (33.3% vs. 19.6%, p ≤ 0.01).
Figure 1.
The percentage of mothers using alcohol, reporting problematic drinking, depressed mood (EPDS > 13), and intimate partner violence (IPV) are plotted over time for mothers living with HIV (MLH) and mothers without HIV (MWOH). Significant differences are indicated at each time point for each variable contrasted by maternal HIV status.
(a) Any alcohol use; (b) Problematic drinking; (c) Depressed mood; (d) IPV
*p < 0.05; **p < 0.01; ***p < 0.001
In contrast to any use of alcohol, we observed that problematic drinking increased over time, showing a significant difference at 36 months (12.2% vs. 5.4%, p = 0.01) and 60 months (17.7% vs. 7.3%, p ≤ 0.01) associated with HIV status. Having a depressed mood or not varied significantly between MLH and MWOH at baseline (42.8% vs. 32.8%, p = 0.04), post-birth (29.0% vs. 19.1%, p = 0.02), 6 months (26.0% vs. 15.9%, p ≤ 0.01), 18 months (20.8% vs. 13.5%, p = 0.04), and 36 months (27.3% vs. 18.1%, p = 0.03) after birth. MLH and MWOH reported similar experiences of IPV throughout the 5 years.
Table 2 compares MLH and MWOH on multiple measures at 60 months and includes unadjusted analyses and adjusted analyses estimates from regression models (estimated mean differences and odds ratios). MLH were significantly more likely to drink any alcohol and to have problematic alcohol use compared to MWOH in the adjusted comparisons. The odds of drinking any alcohol was 3.6 times greater (95% CI: 1.49-8.53) for MLH compared MWOH. Similarly, MLH had a 2.31 times the risk of problematic alcohol use compared to MWOH at 60 months (RR: 2.31, 95% CI: 1.39-3.83). There were no significant differences in depressed mood or IPV between mothers with different HIV status in the unadjusted or adjusted analyses.
Table 2.
Comparison of mothers living with HIV (MLH) and mothers without HIV (MWOH) on alcohol use, problematic drinking, depressed mood (EPDS > 13) and intimate partner violence at 5-years
| MLH (N=153) | MWOH (N=286) | Estimated Odds Ratio or Risk Ratio MLH vs. MWOH | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | OR1 or RR2 | 95% CI | |
| Drinks any alcohol* | 51 | 33.3 | 56 | 19.6 | 3.57a,d,+ | 1.49–8.53 |
| Problematic alcohol use* | 27 | 17.6 | 21 | 7.3 | 2.31a,c,+ | 1.13–3.48 |
| Depressed Mood, EPDS ≥ 13 | 16 | 10.5 | 34 | 11.9 | 0.79a,b | 0.37–1.65 |
| Intimate partner violence | 22 | 14.4 | 34 | 11.9 | 1.15a,d | 0.48–2.76 |
p < 0.05 (t-tests or X2 tests)
p < 0.05 (regression estimates)
Mixed-effects logistic regression for binary outcomes
Risk ratio was reported for problematic alcohol use due to the high prevalence of this outcome
Random-intercept for neighborhood and mother
Random-slope for time using unstructured covariance structure
Random-slope for time using identity covariance structure
Random-slope for time using independent covariance structure
HIV Care of MLH
Engagement in HIV Care for all MLH.
We followed-up 80.0% of MLH (n=143/179) at 5 years. Of those, 86.7% (n=124/143) were engaged in HIV care, 76.9% (n=110/143) reported receipt of ARV, and of those who reported being on ARV treatment, 87.3% (n=96/110) reported being adherent to the medication over the past seven days from 18 months and on.
There was a significant increase at each time point for MLH who were engaged in HIV care: 46.0% (n=64/139) at 6 months, 54.3% (n=75/138) at 18 months, 66.1% (n=84/127) at 36 months, 86.7% (n=124/143) at 60 months (p ≤ 0.01). Also, 152 MLH were engaged in care at some point during the study. However, consistent engagement over time remained a challenge; only 26 MLH were engaged in care at all assessments.
Similarly, there was a significant increase at each time point for MLH who reported receipt of ARV treatment: 30.8% (n=45/146) at 6 months, 47.1% (n=65/138) at 18 months, 52.8% (n=67/127) at 36 months, and 76.9% (n=110/143) at 60 months (p ≤ 0.01). Of those MLH who received ARVs, there was no significant difference in ARV adherence over time: 90.6% (n=58/64) at 18 months, 89.6% (n=60/67) at 36 months, and 87.3% (n=96/110) at 60 months (p = 0.78). Although adherence was above 85% at 18 months and on, only 33 MLH were adherent at all 3 follow-up time points.
Comorbid Effects on HIV Care.
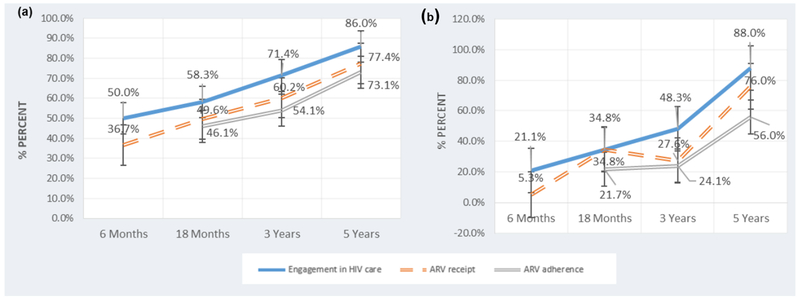
Engaging in HIV care was significantly lower if mothers reported any drinking at 6 months (21.1% vs. 50.0%, p = 0.02), 18 months (34.8% vs. 58.3%, p = 0.04), and 36 months (48.3% vs. 71.4%, p = 0.02) compared to mothers who did not report any alcohol use. Receipt of ARV was significantly lower if mothers drank alcohol at 6 months (5.3% vs. 34.6%, p = 0.01) and 36 months (27.6% vs. 60.2%, p ≤ 0.01). ARV adherence was significantly lower if mothers drank alcohol at 18 months (21.7% vs. 46.1%, p=0.03), 36 months (24.1% vs. 54.1%, p ≤ 0.01), and 60 months (56.0% vs. 73.1%, p = 0.04). We demonstrated these results in Figure 2, where the percentage of MLH who were engaged in HIV care, reported being on ARV treatment, and reported on ARVs with adherence to ARV based on whether the MLH reported using alcohol or not. There were no significant differences in engagement in HIV care, receipt of ARV and ARV adherence based on depressed mood across each of the assessment time points. There were no significant differences in engagement in HIV care if mothers experienced IPV at any assessment time point. However, mothers experiencing IPV were significantly less likely to be receiving ARV at 6 months (12.8% vs. 37.4%, p ≤ 0.01) and reported significantly lower ARV adherence at 60 months (70.6% vs. 90.3%, p = 0.04).
Figure 2.
Maternal self-reports of engagement in HIV Care, receipt of antiretroviral therapies (ARV), and consistent ARV adherence for mothers living with HIV (MLH) who abstain from alcohol use and MLH using alcohol over time.
(a) Engagement in HIV care, ARV receipt, and ARV adherence for MLH who do not use alcohol at 6 months (n=120), 18 months (n=115), 3 years (n=98), and 5 years (n=93)
(b) Engagement in HIV care, ARV receipt, and ARV adherence for MLH who use alcohol at 6 months (n=19), 18 months (n=23), 3 years (n=29), and 5 years (n=50)
DISCUSSION
This longitudinal study of a community-based sample of MLH and MWOH not only provides insight into patterns of alcohol use, depression, and IPV over time among mothers with and without HIV, but it also shows how these co-morbid challenges may influence engagement in HIV care among MLH over time. MLH use alcohol more often, have more problematic alcohol use, and have depressed mood more often than MWOH. Yet, only alcohol use is significantly associated with decreased engagement with HIV care, receipt of ARVs, and ARV adherence. These finding are surprising and point to the importance of alcohol-related interventions for mothers, especially, MLH.
Both MLH and MWOH report the highest rates of alcohol use prior to knowledge of their pregnancy and at five years after delivery. Yet across time, more MLH use alcohol at each time point than MWOH. In addition, there is a significant increase in problematic alcohol use over five years post birth, and a widening gap in problematic alcohol use between MLH and MWOH. Many other studies report on alcohol use as a risk factor for HIV, with women who drink about twice as likely as those who abstain from alcohol to become HIV positive [21, 30, 31]. However, increased risk among women drinking alcohol cannot account for the increase in problematic drinking over time. Additional reasons for increased alcohol consumption among MLH include high levels of perceived HIV-related stigma, mental health problems, and health-related anxiety [32]. In this study, it appears that these negative factors compound over time.
Depressed mood, significantly greater among MLH than MWOH during pregnancy and for three years after birth, is not more common in MLH at five years. This is contrary to previous cross-sectional studies, which have found MLH to be at increased risk of depression [33, 34]. It is possible that the additional challenges of living with HIV, the fear of mother-to-child transmission, and the high rate of new diagnoses during the perinatal period (57%) make MLH more susceptible to depression earlier [33]. Yet, over time this increased risk of depression disappears.
The risk of IPV is greatest in the 12 months prior to pregnancy, when most MLH are unaware of their status. However, contrary to other research, we find that MLH are no more likely to experience IPV than MWOH at any time point over 5 years[17, 35]. Potentially, MLH who are unaware of their HIV status are not yet at elevated risk due to HIV stigma or partner disclosure. Additionally, our questions related to IPV focuses on physical violence within relationships, but did not specifically ask about unwanted or forced sex. In this sample, IPV in South Africa is common (36.5%) and similar for both MLH and MWOH. These findings are similar to other researchers [17, 36, 37].
Our assessments of MLH over time indicate that the 90-90-90 goals set by the global community to eliminate HIV are unlikely to be met. Ensuring that MLH initiate ARV is not a sufficient condition for MLH to thrive; consistent engagement in care is essential. It is unclear if health issues or the Western Cape implementation of Option B+ in 2013 [38] prompted re-engagement; 37.3% of MLH had another child and would have received PMTCT again. However, even when almost all MLH are in care, 23.1% do not receive ARV and 12.7% of those taking ARV are not adhering to treatment. Another recent study, implemented after Option B+, has similar findings: 20% of MLH are not engaged in care and 13% are not virally suppressed at 12 months [11]. Option B+ does not appear to reduce the gap in engagement in care or adherence for many MLH. This may be similar to a pattern seen in the treatment of other chronic diseases: mothers are adherent to medication during pregnancy for the sake of their child, but are not adherent once the baby is born [39].
Interventions to promote consistent HIV care for MLH need to address the comorbid challenges that MLH are facing, particularly alcohol use. As previously observed, alcohol use significantly influences maternal HIV uptake and adherence [20, 21, 40]. We find that MLH who report any alcohol use are about half as likely to engage in HIV care early and remain disengaged from care at later time points. Once in care, MLH who drink alcohol are far less likely to be receiving ARVs. Finally, while over 90% of MLH who do not drink adhere to their medications at all-time points, MLH who drink fluctuate in adherence, falling as low as 63% adherent to ARV. Contrary to previous research, depressed mood does not appear to affect MLH’s HIV care, and IPV has a limited role [41–43].
While there has been a substantial focus globally on addressing mental health care for MLH, [44, 45] the diffusion of alcohol interventions is far lower [46]. Interventions for alcohol use are not broadly implemented in South Africa. Rehabilitation facilities are limited and interventions for alcohol abuse are implemented in primary care facilities which lack the resources and expertise to manage abuse [47].
Paraprofessional home visiting, already being implemented nationally in South Africa, can significantly reduce alcohol use in pregnancy. In fact, among this cohort, perinatal home visiting reduces alcohol use five years post-birth [48]. This study demonstrates significant reductions in alcohol use and abuse five years post-birth [49]. However, these data also suggest that broad implementation of community-wide, structural or policy interventions is warranted.
Limitations
For data on the HIV treatment continuum, we are largely reliant in this study on maternal self-reports. As described in the Methods section, there are three ways that we are able to validate the HIV infections among mothers, suggesting the veracity of maternal reports. In particular, the results of the rapid HIV test are highly consistent with mothers’ self-reports of their HIV status. It would have been desirable to obtain independent observations of medical records, NHLS data, pill counts, or viral load and ARV measures in the blood stream. The HIV rate in this cohort (31%, n=179/584) is consistent with the estimated HIV prevalence for pregnant women in area, which is over 20% for the Cape Metro area as a whole and roughly 34% in the surrounding townships [50]. It is important that we followed a high percentage of the sample over time. Only 22 mothers are not reassessed at least once and 70% are assessed at all-time points.
Similarly, maternal self-reports are used to collect data on alcohol use, depression, and IPV, which may have been underreported due to recall bias and stigma around these issues [32, 24]. However, interviewers in this study are of the same cultural/ethnic background as participants, and the rate of consent to participate (98%) and retention (82.7% at five years) are high, suggesting that women in this study are open to discussing issues related to their health. The PETH alcohol test is a highly accurate measure of alcohol use/abuse, but at over $100 USD, it is not a viable field assessment at this time. A biomarker would have been desirable.
This sample reflects 98% of all pregnant women in 12 neighborhoods and more than 82% are followed across time. Seventy percent of mothers, both MLH and MWOH, completed all assessments from birth to 5 years. The neighborhoods were matched on multiple domains, including formal/informal housing, distance to medical care, HIV care options available, electricity, flush toilets, number of alcohol bars, and were designed to prevent contamination across neighborhoods. These characteristics demonstrate the high internal and external validity in the sample.
CONCLUSION
In the Cape Town townships, MLH are slow to re-engage in HIV care following childbirth. By five years, most MLH are in care, receiving ARV and adherent to their ARV. However, over time, there are high rates of inconsistent retention and adherence. There are few community-based studies of MLH that evaluate the impact of alcohol use, depression, and IPV on HIV care uptake and adherence over time. The current study helps fill this gap and demonstrates how ARV adherence and engagement in HIV care are challenged by alcohol use in particular, especially after the child is born.
Alcohol use remains a significant yet modifiable barrier to consistent engagement in HIV care and ARV adherence. However, it is often overlooked in HIV treatment programs, particularly in LMIC. This study highlights the need for community-level, structural interventions to support MLH in low-resource communities, both during and after pregnancy, and reduce alcohol use and abuse in order to reach the 90-90-90 goals.
Supplementary Material
Acknowledgments
Sources of Support: This work was supported by the National Institute on Alcohol Abuse and Alcoholism (R01AA017104, R24AA022919), National Institute of Mental Health (P30MH058107), Ilifa Labantwana, National Institute of Allergy and Infectious Diseases (AI028697), and the National Center for Advancing Translational Science (UL1TR000124). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
Meetings: To be presented as a poster at the International AIDS Conference in Amsterdam 2018
Conflict of Interest: The authors have no conflicts of interest to declare.
REFERENCES
- 1).Joint United Nations Programme on HIV/AIDS (UNAIDS). The global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva, Switzerland; 2016. Available at: http://www.unaids.org/sites/default/files/media_asset/GlobalPlan2016_en.pdf Accessed January 29, 2019. [Google Scholar]
- 2).Sibanda EL, Weller IV, Hakim JG, Cowan FM. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. Aids. 2013; 27(17):2787–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Clouse K, Pettifor A, Shearer K, et al. Loss to follow‐up before and after delivery among women testing HIV positive during pregnancy in Johannesburg, South Africa. Tropical Medicine & International Health. 2013; 18(4):451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Phillips T, Thebus E, Bekker LG, Mcintyre J, Abrams EJ, Myer L. Disengagement of HIV-positive pregnant and postpartum women from antiretroviral therapy services: a cohort study. Journal of the International AIDS Society. 2014; 17(1):19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Haas AD, Tenthani L, Msukwa MT, et al. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi’s option B+ programme: an observational cohort study. The Lancet HIV. 2016; 3(4):e175–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Woelk GB, Ndatimana D, Behan S, et al. Retention of mothers and infants in the prevention of mother-to-child transmission of HIV programme is associated with individual and facility-level factors in Rwanda. Journal of the International AIDS Society. 2016; 19(5Suppl 4):20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Gumede-Moyo S, Filteau S, Munthali T, Todd J, Musonda P. Implementation effectiveness of revised (post-2010) World Health Organization guidelines on prevention of mother-to-child transmission of HIV using routinely collected data in sub-Saharan Africa: A systematic literature review. Medicine. 2017; 96(40):e8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Decker S, Rempis E, Schnack A, et al. Prevention of mother-to-child transmission of HIV: Postpartum adherence to Option B+ until 18 months in Western Uganda. Plos One. 2017; 12(6):e0179448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Myer L, Phillips TK, Hsiao NY, et al. Plasma viraemia in HIV-positive pregnant women entering antenatal care in South Africa. Journal of the International AIDS Society. 2015; 18(1):20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Myer L, Dunning L, Lesosky M, et al. Frequency of viremic episodes in HIV-infected women initiating antiretroviral therapy during pregnancy: a cohort study. Clinical Infectious Diseases. 2017; 64(4):422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Myer L, Phillips TK, Zerbe A, et al. Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial. PLoS medicine. 2018; 15(3):e1002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland; 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf Accessed January 29, 2019. [Google Scholar]
- 13).Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS research and human retroviruses. 2010; 26(5):511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Boarts JM, Sledjeski EM, Bogart LM, Delahanty DL. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS and Behavior. 2006; 10(3):253–261. [DOI] [PubMed] [Google Scholar]
- 15).Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004; 103(4):698–709. [DOI] [PubMed] [Google Scholar]
- 16).Devries KM, Mak JYT, García-Moreno C, et al. The Global Prevalence of Intimate Partner Violence Against Women. Science. 2013; 340(6140):1527–1528. [DOI] [PubMed] [Google Scholar]
- 17).Jewkes R, Dunkle K, Nduna M, Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. The Lancet. 2010; 376(9734):41–48. [DOI] [PubMed] [Google Scholar]
- 18).Kiene SM, Lule H, Sileo KM, Silmi KP, Wanyenze RK. Depression, alcohol use, and intimate partner violence among outpatients in rural Uganda: vulnerabilities for HIV, STIs and high risk sexual behavior. BMC infectious diseases. 2017; 17(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Jasseron C, Mandelbrot L, Dollfus C, et al. Non-disclosure of a pregnant woman’s HIV status to her partner is associated with non-optimal prevention of mother-to-child transmission. AIDS and Behavior. 2013; 17(2):488–497. [DOI] [PubMed] [Google Scholar]
- 20).Magidson JF, Saal W, Nel A, Remmert JE, Kagee A. Relationship between depressive symptoms, alcohol use, and antiretroviral therapy adherence among HIV-infected, clinic-attending patients in South Africa. Journal of Health Psychology. 2016; 22(11):1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Morajele N, Kekwaletswe C, Nkosi S. Associations between alcohol use, other psychosocial factors, structural factors and antiretroviral therapy (ART) adherence among South African ART recipients. AIDS and Behavior. 2014; 18(3):519–524. [DOI] [PubMed] [Google Scholar]
- 22).Seth P, Kidder D, Pals S, et al. Psychosocial functioning and depressive symptoms among HIV-positive persons receiving care and treatment in Kenya, Namibia, and Tanzania. Prev Sci. 2014;15(3):318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).(BLIND FOR REVIEW), le Roux IM, (BLINDED FOR REVIEW), et al. Philani Plus (+): a Mentor Mother community health worker home visiting program to improve maternal and infants’ outcomes. Prevention Science. 2011;12(4):372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).South Africa National Department of Health. Policy and Guidelines of the Implementation of the PMTCT Programme. 2008. Aavailable at: https://www.ilo.org/wcmsp5/groups/public/---ed_protect/---protrav/---ilo_aids/documents/legaldocument/wcms_125633.pdf Accessed January 29, 2019.
- 25).South Africa National Department of Health. South African Antiretroviral Treatment Guidelines. 2010. Available at: http://apps.who.int/medicinedocs/documents/s19153en/s19153en.pdf Accessed January 29, 2019.
- 26).Tsai AC, Bangsberg DR, Emenyonu N, Senkungu JK, Martin JN, Weiser SD. The social context of food insecurity among persons living with HIV/AIDS in rural Uganda. Social science & medicine. 2011; 73(12):1717–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT, the alcohol use disorders identification test: Guidelines for use in primary care. Genevea, Switzerland: World Health Organization, Dept. of Mental Health and Substance Dependence; 2001. https://apps.who.int/iris/bitstream/handle/10665/67205/WHO_MSD_MSB_01.6a.pdf;jsessionid=A0895B346180CE265D51A032E7901B92?sequence=1 Accessed January 29, 2019. [Google Scholar]
- 28).Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression - Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry. 1987; 150:782–786. [DOI] [PubMed] [Google Scholar]
- 29).Lawrie TA, Hofmeyr GJ, de Jager M. Validation of the Edinburgh Postnatal Depression Scale on a cohort of South African women. The South African Medical Journal. 1998; 88(10):1340–1344. [PubMed] [Google Scholar]
- 30).Fisher JC, Bang H, Kapiga SH. The association between HIV infection and alcohol use: a systematic review and meta-analysis of African studies. Sexually transmitted diseases. 2007;34(11):856–863. [DOI] [PubMed] [Google Scholar]
- 31).Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings. Prevention science. 2007;8(2):141. [DOI] [PubMed] [Google Scholar]
- 32).Murphy DA, Austin EL, Greenwell L. Correlates of HIV-Related Stigma Among HIV-Positive Mothers and Their Uninfected Adolescent Children. Women & Health. 2007;44(3):19–42. [DOI] [PubMed] [Google Scholar]
- 33).Kaida A, Matthews LT, Ashaba S, et al. Depression During Pregnancy and the Postpartum Among HIV-Infected Women on Antiretroviral Therapy in Uganda. J Acquir Immune Defic Syndr. 2014;67(4):179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Kapetanovic S, Dass-Brailsford P, Nora D, Talisman N. Mental Health of HIV-Seropositive Women During Pregnancy and Postpartum Period: A Comprehensive Literature Review. AIDS and Behavior. 2015;18(6):1152–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Li Y, Marshall CM, Rees HC, Nunez A, Ezeanolue EE, Ehiri JE. Intimate partner violence and HIV infection among women: a systematic review and meta‐analysis. Journal of the international AIDS society. 2014;17(1):18845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Russell BS, Eaton LA, & Petersen-Williams P (2013). Intersecting epidemics among pregnant women: alcohol use, interpersonal violence, and HIV infection in South Africa. Current HIV/AIDS Reports, 10(1), 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Gibbs Andrew, Dunkle Kristin, Willan Samantha, Jama-Shai Nwabisa Washington Laura, and Jewkes Rachel. “Are women’s experiences of emotional and economic intimate partner violence associated with HIV-risk behaviour? A cross-sectional analysis of young women in informal settlements in South Africa.” AIDS care 31, no. 6 (2019): 667–674. [DOI] [PubMed] [Google Scholar]
- 38).Burton R, Giddy J, Stinson K. Prevention of mother-to-child transmission in South Africa: an ever-changing landscape. Obstetric medicine. 2015;8(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Matsui D Adherence with drug therapy in pregnancy. Obstetrics and gynecology international. 2012; 2012. doi: 10.1155/2012/796590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL. The impact of alcohol use and related disorders on the HIV continuum of care: a systematic review. Current HIV/AIDS Report. 2015;12(4):421–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC medicine. 2014;12(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Loftus H, Burnett A, Naylor S, Bates S, Greig J. HIV control in postpartum mothers: a turbulent time. International journal of STD & AIDS. 2016;27(8):680–683. [DOI] [PubMed] [Google Scholar]
- 43).Hatcher AM, Stöckl H, Christofides N, et al. Mechanisms linking intimate partner violence and prevention of mother-to-child transmission of HIV: A qualitative study in South Africa. Social Science & Medicine. 2016;168:130–139. [DOI] [PubMed] [Google Scholar]
- 44).Barry MM, Clarke AM, Jenkins R, Patel V. A systematic review of the effectiveness of mental health promotion interventions for young people in low and middle income countries. BMC Public Health. 2013;13:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Bhana A, Mellins CA, Petersen I, et al. The VUKA family program: piloting a family-based psychosocial intervention to promote health and mental health among HIV infected early adolescents in South Africa. AIDS Care. 2014;26(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Carrasco MA, Esser MB, Sparks A, Kaufman MR. HIV-alcohol risk reduction interventions in Sub-Saharan Africa: a systematic review of the literature and recommendations for a Way forward. AIDS and Behavior. 2016; 20(3):484–503. [DOI] [PubMed] [Google Scholar]
- 47).Parry CD. South Africa: alcohol today. Addiction. 2005;100(4):426–429. [DOI] [PubMed] [Google Scholar]
- 48).(BLIND FOR REVIEW). (2018, July) South African Mothers Living with HIV (MLH) do not sustain HIV care 5 years post-birth, especially when problematic alcohol use occurs. International AIDS Society, Amsterdam. [Google Scholar]
- 49).(BLIND FOR REVIEW) Evaluating the Philani Project. Meeting of Health Care Funders in South Africa, held at the Philani Organization in Khayelitsha; 2018; Cape Town. [Google Scholar]
- 50).National Department of Health. The 2012 National Antenatal Sentinel HIV & Herpes Simplex Type-2 Prevalence Survey in South Africa. Pretoria, South Africa: National Department of Health, 2014. Available at: https://www.health-e.org.za/wp-content/uploads/2014/05/ASHIVHerp_Report2014_22May2014.pdf Accessed January 29, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.