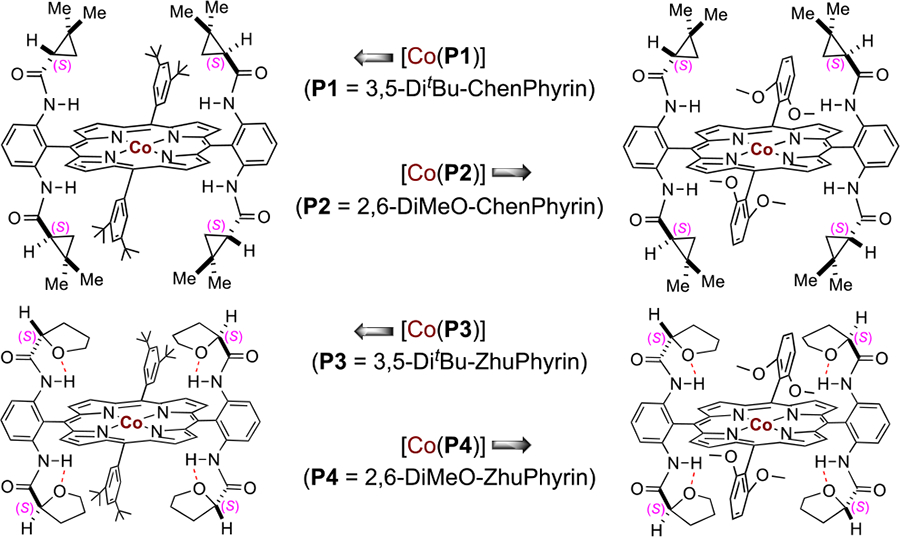
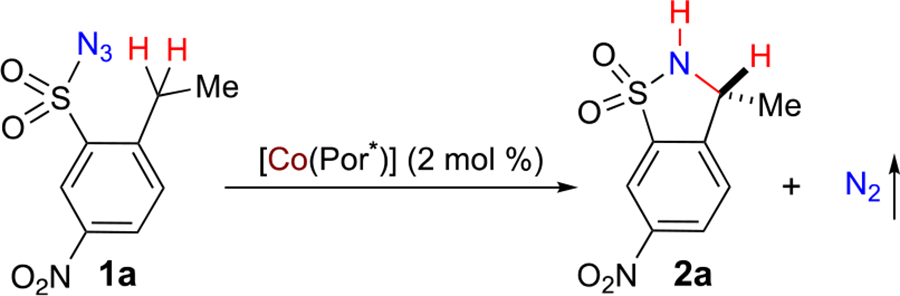
Table 1.
Ligand Effect on Co(II)-Based Metalloradical System for Asymmetric 1,5-C–H Amination of Arylsulfonyl Azidea
 | |||||
|---|---|---|---|---|---|
| entry | catalyst | temp (°C) | solvent | yield (%) | ee (%) |
| 1 | [Co(P1)] | 80 | chlorobenzene | 99 | 28 |
| 2 | [Co(P2)] | 80 | chlorobenzene | 99 | 32 |
| 3 | [Co(P3)] | 80 | chlorobenzene | 99 | 49 |
| 4 | [Co(P4)] | 80 | chlorobenzene | 99 | 84 |
| 5 | [Co(P4)] | 60 | chlorobenzene | 99 | 86 |
| 6 | [Co(P4)] | 40 | chlorobenzene | 63 | 88 |
| 7 | [Co(P4)] | 60 | chloroform | 70 | 89 |
| 8b | [Co(P4)] | 50 | chloroform | 96 | 92 |
Reactions were carried out for 18 h on 0.10 mmol scale under N2; [1a] = 0.25 M. Isolated yields Enantiomeric excess determined by chiral HPLC.
4 mol % catalyst for 48 h.